Abstract
Despite the pivotal role of stannum doping in achieving ultrahigh piezoelectric performance in barium titanate-based ceramics, the fundamental mechanisms underlying this enhancement remain elusive. Here, we introduce a single variable nonstoichiometric stannum strategy in lead-free barium titanate-based ceramics with giant piezoelectricity, revealing that stannum doping contributes intrinsically and extrinsically to enhance piezoelectricity. Density functional theory calculations elucidate the intrinsic enhancement of polarization arising from lattice distortion and increased space for titanium-oxygen bonds induced by optimal stannum doping, which is corroborated by Rayleigh analysis. A phase transition from ferroelectric multiphase coexistence to paraelectric phase is observed, alongside a rapid miniaturized and eventually disappeared domains with increasing stannum doping. This evolution in phase structure and domain configuration induces a nearly vanishing polarization anisotropy and low domain wall energy, facilitating easy polarization rotation and domain wall motion, thereby significantly contributing to the extrinsic piezoelectric response. Consequently, the origins of ultrahigh performance can be attributed to the synergistic effect of stannum-induced intrinsic and extrinsic contributions in barium titanate-based ceramics. This study provides fundamental insights into the role of doping elements and offers guidance for the design of high-performance piezoelectrics.
Similar content being viewed by others
Introduction
Perovskite ferroelectrics, renowned for converting mechanical energy into electrical energy and vice versa, serve as foundational materials across various applications, including military actuators, electronic sensors, and medical ultrasonic transducers1,2,3,4,5,6,7,8,9. Nowadays, the global market for piezoelectric and ferroelectric devices is projected to expand into a multi-billion-dollar industry, with an annual growth rate of 13.2%6. High-performance lead zirconate titanate [Pb(Zr, Ti)O3, PZT]-based piezoelectric ceramics have been employed extensively in piezoelectric devices due to their superior piezoelectric coefficients and high Curie temperature, thereby dominating the piezoelectric ceramics market10. However, the societal ramifications of lead pollution, such as cancer, fetal malformations, and food safety concerns, are becoming increasingly severe10,11,12. In pursuit of sustainable development, legislation and regulations have been enacted or strengthened globally in response to escalating ecological and environmental concerns, thereby directing significant attention toward the development of lead-free piezoelectric materials3,4,5,11.
As the earliest discovered perovskite-type lead-free ferroelectrics, barium titanate (BaTiO3, BT)-based ceramics have been extensively studied for their remarkable properties, particularly their piezoelectric performance10,12,13,14,15,16,17,18,19,20,21. To meet specific device requirements, chemical modification is the most commonly employed method to tailor the electrical properties of BT-based ceramics12. The hybridization between B-site atoms and oxygen atoms is fundamentally responsible for the ferroelectricity observed in BT-based ceramics22. The associated physical characteristics, such as lattice distortion, domain configuration, and phase boundary, can be modulated through chemical substitution or doping12. For example, high piezoelectricity can be achieved through ions doping to shift the phase-transition temperature and form the multiphase boundary. The increase in dielectric permittivity, hierarchical domain structure, and more directions of polarization rotation after chemical substitution or doping are identified as the origins of enhanced piezoelectricity near the phase boundary where the polarization vectors can be easily rotated due to the low energy barrier3,9,12. This substitution typically involves replacing cations at either the A or B sites within the BT-based ceramics. Low-valence ions are commonly incorporated into the A site, displacing Ba²⁺ ions, while high-valence ions are generally introduced into the B site to replace Ti4⁺ ions. To achieve desirable piezoelectric properties, isovalent substitution has proven to be the most effective strategy for altering the structural and functional attributes of BT-based ceramics10,12,13,14,15,16,17,18,19,20,21. The most frequently utilized cations for substitution at the Ba²⁺ site are Ca²⁺ or Sr²⁺, while valence-stable Zr4⁺, Hf4⁺, and Sn4⁺ cations are preferred for substituting Ti4⁺ at the B site due to their comparable ionic valence and radii, as shown in Fig. 1a. The construction of a multiphase boundary has garnered significant attention, as the isovalent substitution of Zr4⁺, Hf4⁺, and Sn4⁺ for Ti4⁺ significantly enhances the piezoelectric properties of BT-based ceramics12.
a Schematic of B-site doping in ABO3 structure. b Relative dielectric permittivity-temperature (εr-T) curves and phase diagram. c Increasing speed of phase transition temperatures from rhombohedral to orthorhombic phases (TR-O), from orthorhombic to tetragonal phases (TO-T), and Curie temperature (TC) for B-site Zr4+, Hf4+, or Sn4+ doped BT ceramics12. d Piezoelectric coefficient (d33) of BT and BT-based ceramics with B-site Zr4+, Hf4+ or Sn4+ substitution. e d33/190 pC/N for BT and BT-based ceramics with B-site Sn4+ doping. f Schematic of contribution on ultrahigh piezoelectric performance for Sn doping in BT-based ceramics.
BT ceramics undergo three distinct phase transitions—tetragonal-cubic (T-C), orthorhombic-tetragonal (O-T), and rhombohedral-orthorhombic (R-O)—as the temperature decreases from high to low9. The introduction of Zr4⁺, Hf4⁺, and Sn4⁺ into the B site of BT shifts the R-O transition temperature (TR-O) and the O-T transition temperature (TO-T) to higher temperatures while simultaneously decreasing the T-C transition temperature (TC), as illustrated in Fig. 1b. The doping of Zr⁴⁺ and Hf⁴⁺ exhibits similar effects on shifting the phase-transition temperatures (TR-O, TO-T, and TC) of BT-based ceramics. However, the rate of increase in TR-O and TO-T induced by Sn⁴⁺ substitution is slower compared to that of Zr⁴⁺ or Hf⁴⁺ substitution, whereas the decrease in TC is more pronounced with Sn⁴⁺ doping. To quantitatively describe the impact on phase boundary shifts, the average rates of change for TR-O, TO-T and TC with Zr⁴⁺, Hf⁴⁺, or Sn⁴⁺ substitution are plotted in Fig. 1c. Specifically, TR-O increases by approximately 11.8 °C per mol% and TO-T by around 5.0 °C per mol%, while TC decreases by approximately 4.6 °C per mol% with the moderate substitution of Zr⁴⁺ or Hf⁴⁺ at the B site. Interestingly, Sn⁴⁺ substitution at the B site results in the improvement by approximately 9.7 °C per mol% for TR-O and 2.1 °C per mol% for TO-T, and the descent by 7.7 °C per mol% for TC in BT ceramics12. Based on the effects of Zr⁴⁺, Hf⁴⁺, and Sn⁴⁺ substitutions at the B site, an ideal multiphase boundary can be successfully constructed to improve piezoelectric properties12,18. Figure 1d presents the piezoelectric properties of various BT-based ceramics with Zr⁴⁺, Hf⁴⁺, or Sn⁴⁺ substitution, which are comparable to those of traditional lead-based piezoelectric ceramics. All the BT-based ceramics with chemical substitution exhibit a higher piezoelectric property than that of pure BT ceramics, particularly for the ceramics with co-substitution at A/B sites (A site: Ca²⁺, Sr²⁺; B site: Zr⁴⁺, Hf⁴⁺, and Sn⁴⁺)12,15,16,17,18,19,20,21. Although Zr⁴⁺, Hf⁴⁺, and Sn⁴⁺ substitutions enhance the piezoelectric properties, Sn⁴⁺ is the most effective dopant at the B site in BT-based ceramics, as shown in Fig. 1e. Nevertheless, the origin of this ultrahigh performance in Sn-doped BT-based piezoelectrics remains unclear. Therefore, understanding how Sn dopants contribute to enhancing piezoelectric properties intrinsically and extrinsically remains a critical area for further research (See Fig. 1f).
To this end, a kind of BT-based composition with A/B site co-substitution (A site: Sr2+; B site: Sn4+) locates at the multiphase boundary, achieving a high piezoelectricity d33 up to 850 pC N−1. To reveal the contribution of Sn dopant on ultrahigh-performance BT-based piezoelectrics, a nonstoichiometric strategy with a single variable was implemented in Ba0.86Sr0.14Ti0.92Sn0.08+x (x = −0.02, 0, and 0.02; abbreviated as BSTS0.08+x). Our study integrates high-resolution transmission electron microscopy (TEM), piezoresponse force microscopy (PFM), density functional theory (DFT), and Rayleigh analysis to examine the contribution to piezoelectric performance in three pre-selected compositions with varying Sn content. Here, the extrinsic contribution mainly results from the domain switching and domain wall motion, and the contribution by phase boundary. The intrinsic piezoelectric response related to the lattice distortion contributes to the intrinsic contribution9. The increased intrinsic contribution arises from the enhancement of polarization, resulting from lattice distortions and increased space for Ti-O bonds in the ceramics with a small amount of Sn doping. The domain becomes quite small as the phase transitions from O-T to R-O-T, facilitating the piezoelectricity due to the easy domain wall motion under an external field. Benefiting from the synergy of intrinsic and extrinsic contributions induced by Sn doping, an ultrahigh d33 is obtained. This work offers fundamental insights for designing high-performance piezoelectric materials through chemical modification.
Results
Structure and nonstoichiometric characterization
In view of the ionic radius difference between Ti⁴⁺ (0.605 Å, CN = 6) and Sn⁴⁺ (0.69 Å, CN = 6), it is essential to analyze the stability of the perovskite structure after doping. The stability can be assessed using the tolerance factor (τ), defined by the following formula23,24:
where τ is the tolerance factor, and RA, RB, and RO are the ionic radii of the A-site, B-site, and oxygen ions in the ABO3 perovskite structure, respectively. For a complex perovskite, the values for RA and RB are normalized by the atomic ratio. The average ionic radius at the A site and B site can be calculated using the following equations:
where the ionic radius of Ba2+ and Sr2+ at A site (CN = 12) are 1.61 Å and 1.44 Å, respectively. The radius of Ti4+ and Sn4+ at B site (CN = 6) are 0.605 Å and 0.69 Å, and the radii of O2− is 1.40 Å. Notably, all τ remains approximately 1 for all compositions (τ−0.02: 1.057, τ0.00: 1.0496, τ0.02: 1.042), indicating that the BSTS0.08+x ceramics exhibit the high stability of ABO3 structure23,24.
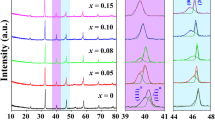
A typical perovskite structure is achieved without any secondary phases in BSTS0.08+x ceramics (See Figs. S1 and S2). Figure 2a illustrates the XRD patterns of BSTS0.08+x ceramics. The characteristic XRD peak positions shift towards lower angles with increasing Sn⁴⁺ concentration in the range of −0.02 ≤ x ≤ 0.02, indicating lattice expansion due to Sn⁴⁺ incorporation. The minimal lattice parameters observed at x = −0.02 can be attributed to the formation of B-site vacancies caused by B-site deficiency25. Conversely, the expanded lattice parameters are primarily due to the substitution of smaller-radius Ti⁴⁺ ions with larger-radius Sn⁴⁺ ions at B site26. The enlarged Raman spectra around 515 cm⁻¹ are also presented in Fig. 2a, featuring two peaks corresponding to the E and A1 modes. Both E and A1 peaks shift to lower wavenumbers regardless of the deficiency or excess of Sn⁴⁺ ions at B site, which can be explained by the weakening of the B-O bond in the ceramics26,27,28. A shift to higher wavenumbers in a Raman mode indicates a stronger chemical bond, while a shift to lower wavenumbers signifies a weaker bond. Consequently, the observed attenuation of the wavenumber at x = −0.02 can be attributed to the formation of vacancies at B site. Furthermore, the downshift in the spectra results from the weakening of the B-O bond when Sn⁴⁺ substitutes for Ti⁴⁺, due to their differing electronegativities (Sn: 1.96, Ti: 2.04). Additionally, the disparity in ionic radii between Sn⁴⁺ and Ti⁴⁺ induces lattice distortion in the ceramics, further contributing to the weakening of the B-O bond. Thus, the observed shifts of the E and A1 peaks to lower wavenumbers corroborate the hypothesis that an excess of Sn⁴⁺ replaces Ti⁴⁺ (x = 0.02).
a X-ray diffraction (XRD) patterns and Raman spectra. b X-ray photoelectron spectroscopy (XPS) spectra of Ti and Sn. c Intensity ratio of Ti and Sn. d Electron probe microanalyzer (EPMA) elements mapping of Ti and Sn. e Statistical atomic percentage of the experimental (expe) and theoretical (theo) results, and the difference (D) for Ti and Sn. f Impedance spectra, and g the activation energy of grain (Eag) and grain boundary (Eagb) for BSTS0.08+x ceramics.
To further elucidate the impact of excess Sn⁴⁺ on the structure, XPS patterns of Ti 2p and Sn 3d are presented in Fig. 2b. XPS spectra are extensively employed to provide quantitative information on the concentration of specific atoms29,30,31. By calculating the relative content based on the peak area ratio, the proportions of Ti⁴⁺ and Sn⁴⁺ ions can be determined32,33. Using x = 0 as the normalization standard, the content ratio of Ti⁴⁺ and Sn⁴⁺ are depicted in Fig. 2c. Notably, the results illustrate that the Ti⁴⁺ content decreases while the Sn⁴⁺ content increases from x = −0.02 to x = 0.02, thereby corroborating the results conforming to the designed chemical formula. The stoichiometric ratio of Ti and Sn elements is of critical importance for elucidating the relationship between structure and property, which is further analyzed using an Electron Probe Micro-Analyzer (EPMA)34. The backscattered electron image (BEI) presents a dense structure in polished samples (see Fig. S3a). Figure 2d reveals no elemental segregation in ceramics with x ≤ 0. However, the Ti element aggregation is observed in ceramics with x > 0, identifiable by the significant color contrast in the elemental mapping of Ti and Sn. The atomic percentage of the four elements (Ba, Sr, Ti, Sn) is presented in Fig. 2e and Table S1. The discrepancies between theoretical and experimental results are all within 1%, indicating excellent agreement with the stoichiometric composition of BSTS0.08+x ceramics, particularly for Ti and Sn. Additionally, BSTS0.08+x ceramics show a large but similar average grain size (see Fig. S3b and Table S1), thus the grain size effect on piezoelectric property of these ceramics is insignificant.
To further elucidate the impact of nonstoichiometry on conductivity mechanisms, impedance spectroscopy was employed. The complex impedance spectra are plotted in Fig. 2f, S4a and S4b. The impedance response is bifurcated into two components: the high-frequency grain response and the low-frequency grain boundary response, attributable to the distinct relaxation times of grain and grain boundary35,36. The radius of the semicircles decreases with increasing temperature, which is indicative of charge carrier accumulation near the grain boundaries, thereby reducing resistivity. For a quantitative analysis of the contributions from the grain and grain boundary, the Z’-Z” curves were fitted using an equivalent circuit model (See Fig. S4c). The activation energy (Ea) was estimated using the following equation:
where σ0 is the temperature-independent conductivity, k is the Boltzmann constant, and T is the absolute temperature. The activation energy for the grain (Eag) and grain boundary (Eagb) can be obtained according to the equation, as is shown in Fig. 2g. It is documented that the Ea of A-site cations, B-site cations, and oxygen vacancies are approximately 12 eV, 4 eV, and less than 2 eV, respectively32,37,38. The calculated Eag (6.36–6.43 eV) and Eagb values (4.09–6.029 eV) closely align with the Ea of B-site cations, corroborating the substitution of Ti⁴⁺ with Sn⁴⁺ at B site. The reduced Eag is intimately connected to defects arising from the nonstoichiometric effect. A higher Eag at x = −0.02 is due to defects restricting the migration of free charge carriers within the grain. Additionally, the larger Eagb can be attributed to the accumulation of Ti⁴⁺ at the grain boundary, replaced by the excess Sn⁴⁺ in the ceramics with x = 0.02. Ti⁴⁺ at the grain boundary is less mobile under an electric field, resulting in a higher Eagb. The presence of defects or elements enrichment is not conducive to obtaining high piezoelectricity since they may impede the polarization rotation and domain wall motion33.
Macro-performance and extrinsic contribution
As discussed above, Sn⁴⁺ doping can elevate the TR-O and TO-T while concurrently lowering the TC. The nonstoichiometric effect of Sn⁴⁺ plays a pivotal role in phase engineering within these ceramics. Figure 3a illustrates the εr-T curves of BSTS0.08+x ceramics. In the ceramics with x ≤ 0, three distinct dielectric peaks are evident, corresponding to the TC, TR-O, and TO-T as the temperature decreases. Conversely, a single dielectric anomalous peak (TC) is observed in the ceramics with x > 0. The phase transition temperatures for BSTS0.08+x ceramics can be extracted from the εr-T curves (See Table S2). It indicates that a progression phase transition from O-T to a R-O-T phases coexistence, and ultimately to the C phase. The multiphase coexistence can contribute to high piezoelectric property, particularly for the R-O-T phase coexistence3,9,12. To further confirm the evolution of the phase structure, Rietveld refinement was conducted using R (R3m), O (Amm2), T (P4mm), and C (Pm3m) crystal symmetries (see Fig. S5). The refinement results exhibit high reliability, as evidenced by the low Sig (1.27–1.54) and Rwp (6.51–7.71%), with the corresponding structural parameters showing in Table S3. These results corroborate the transformation of phase structure in the ceramic. Additionally, an increase in cell volume is observed in the ceramics with higher Sn content, consistent with the low-angle shift in the XRD patterns. This phenomenon is attributed to the substitution of Ti⁴⁺ by Sn⁴⁺ at B site.
a Relative dielectric permittivity-temperature (εr-T) curves; b polarization-electric field (P-E) loops; c strain-electric field (S-E) curves; d piezoelectric coefficient (d33); e piezoresponse force microscopy (PFM) amplitude images; f transmission electron microscopy (TEM) images; g statistics of domain width of representative domains marked in TEM images; h domain width of BSTS0.08+x ceramics.
Figure 3b, c depicts the P-E and S-E loops of the ceramics, measured at 1 Hz and 30 kV/cm. The typical hysteresis loops at x ≤ 0 indicate that the ceramics exhibit ferroelectric behavior. Conversely, the ceramics with x > 0 display slender P-E and S-E loops, characteristic of a paraelectric phase. The shape of the P-E and S-E loops can be further quantified by the squareness ratio (Rsq) asymmetry (γs), calculated as follows39,40:
where Pr is the remanent polarization, Ps is the saturation polarization, P1.1Ec is the polarization at 1.1 times the coercive field (Ec), and ΔS− and ΔS+ are the poling strain of the left and right sides of the S-E loops, respectively. It is reported that a value of 2 is obtained for perfectly rectangular polarization loops; thus, the decreased Rsq with increasing Sn indicates degraded rectangular P-E loops40. The γs remains around 0, suggesting good symmetry of S-E loops (See Table S4). The related eigenvalues, including Ps, Pr, Ec, positive strain (Spos), and negative strain (Sneg), are presented in Table S4. All values decrease with increasing Sn content, which is mainly attributed to the evolution of the phase structure and domain size. Remarkably, the piezoelectric coefficient (d33) is significantly enhanced from 432 pC/N to 825 pC/N with increasing Sn from −0.02 to 0.00, and then sharply decreases with excess Sn doping, as shown in Fig. 3d. According to the theoretical relation d33 ~ εrPr, the piezoelectric property is in direct proportion to the product of relative dielectric permittivity and remnant polarization. The variation tendency of d33 is highly consistent with εrPr (See Fig. S6), implying that the high dielectric and ferroelectric properties play a crucial role in enhancing the piezoelectric performance41,42.
In addition, the domain morphology as well as switching behavior is explored, as shown in Fig. 3e, f. Combining with the amplitude and phase images (Fig. 3e and S7), the miniaturized domain structure with increasing Sn4+ content is observed (See Fig. 3e), which is also ascribed to the evolution of phase structure. Meanwhile, an optimized piezoelectric response is also gained at x = 0 (the insert of Fig. 3e). It is mainly generated by the modified phase content with a lowered energy barrier, which is also the origin of high piezoelectric performance43. Based on the PFM and TEM domain images, a stripe ferroelectric domain pattern and obvious non-180° domains are observed in the ceramics with x = −0.02, indicating strong ferroelectric and strain properties in these ceramics43. In contrast, a mottled domain pattern, consisting of numerous nanoscale domains, is observed in the ceramics (x = 0) along with uniformly distributed elements (See Fig. 3f and S8). This observation suggests the elimination of polarization anisotropy, consistent with high-piezoelectric BT-based ceramics reported elsewhere13,14. As Sn content increases, a paraelectric phase devoid of any discernible domains is observed in the ceramics. The evolution of domains is further confirmed by regular intensity fluctuations in microregions marked by dotted lines (See Fig. 3g), with domain size decreasing from 178 nm for x = −0.02 to 37 nm for x = 0, and eventually disappearing (See Fig. 3h). The relationship between piezoelectric property (d33) and domain size (wd) follows the formula44:
where a, b, and n are the constants specific to the piezoelectric material. According to this formula, d33 significantly increases as the domain size decreases from 178 nm to 37 nm when composition changes from x = −0.02 to x = 0, aligning closely with the evolution of d33 observed in the ceramics. Consequently, as Sn content increases, the extrinsic contribution contributed by domain and phase structures increases from x = −0.02 to x = 0 and then decreases at x = 0.02.
Intrinsic contribution
It is well-known that the piezoelectric response, similar to the dielectric response under an external electric field, typically exhibits nonlinear and hysteretic characteristics43. This behavior arises from the composite nature of the piezoelectric response, which includes both intrinsic and extrinsic contributions. To further elucidate the origin of the intrinsic contribution, the Sn-doped BT ceramics were studied by the density functional theory (DFT) calculations, as depicted in Fig. 4a, b. The Bader charge and charge transfer for Ti are smaller than those for Sn (See Fig. 4a, b, Tables S5–S7). Conversely, the Born effective charge of Ti (ZTi* ≈ +7.45) is substantially larger than that of Sn (ZSn* ≈ +4.42), as shown in Fig. 4b. These results suggest that the orbital hybridization between Sn and oxygen atoms is significantly weaker than that between Ti and oxygen atoms. It is well-documented that hybridization between B-site atoms and oxygen atoms mitigates short-range repulsions, facilitating off-center ion displacements and thereby generating dipoles in each unit cell of a conventional ferroelectric14. Subsequently, the domain structure is established through the interaction of these dipoles under a long-range Coulomb field. When Sn atoms replace Ti atoms in the unit cells, the dipole formation is markedly hindered, leading to the disruption of the typical ferroelectric domain structure in BT ceramics. This phenomenon aligns with observations reported elsewhere14,45. The lattice volume increases as the concentration of Sn increases due to the larger ionic radius of Sn ions (0.69 Å, CN = 6) substituting the smaller Ti ions (0.605 Å, CN = 6) in BT ceramics (see Tables S8 and S9). This volumetric expansion is corroborated by the XRD patterns. Stress is induced within the matrix by the smaller lattice constants associated with lower Sn concentration in BT ceramics. This stress can expand the polar nanoregions, significantly influencing the ferroelectric properties of the materials14. It is reported that the dielectric properties can be greatly strengthened by the mismatch strain due to the different substrates exhibiting different phases in BaTiO3 thin films46. Notably, increased mismatch strain markedly improves the coercive force and saturation polarization. Here, the stress on the nanoscale domains is three-dimensional, yet the resultant effect is similar. This stress induces an expansion of the unit cells within the nanoscale domains in all three dimensions, creating more space between the Ti atoms and the oxygen octahedra. This increased space facilitates larger off-center displacements, which are directly related to polarization. Consequently, this expansion enhances polarization within the nanoscale domains. To verify the enhanced polarization through theoretical calculations, the soft phonon mode (Γ4-) corresponding to the origin of ferroelectricity and the paraelectric-ferroelectric phase transition is examined in Fig. 4c. The Γ4- mode initially strengthens in BT ceramics with a small amount of Sn but diminishes with further doping. The enhancement of Γ4- is attributed to increased polarization induced by lattice distortions and the additional space for Ti-O bonds in BT ceramics with a small amount of Sn. Conversely, more Sn doping disrupts the long-range order, leading to decreased Γ4- and reduced polarization in BT ceramics. As a result, the enhancement of polarization is contributed intrinsically after a small amount of Sn doping, then enhancing the piezoelectric properties.
a Charge density distribution; b Born effective charge and charge transfer of Ti and Sn in BaTiO3 and BaSnO3. c Soft phonon mode (Γ4-) of Ba(Ti1-xSnx)O3. d Subcoercive measurements of the relative dielectric permittivity (εr) for BSTS0.08+x ceramics with different frequencies. e Initial permittivity (εinit); f Rayleigh coefficient (α); g evolution of intrinsic contribution for BSTS0.08+x ceramics.
Given the parallel origins of piezoelectric and dielectric properties, the evolution of intrinsic and extrinsic contributions in dielectric behavior is similarly applicable to piezoelectric performance. To delineate the reversible and irreversible contributions on dielectric activities, Rayleigh analysis was employed. The electric-field dependence of the dielectric permittivity [ε(E)] of BSTS0.08+x ceramics is illustrated in Fig. 4d, measured under subcoercive conditions in the low-field regime (≤0.8 kV/cm), where domain structure and domain wall density remain unchanged. The ε(E) increases monotonically with applied electric fields at different frequencies (100 Hz, 1000 Hz, and 10000 Hz), consistent with the Rayleigh relationship13,16,40:
where ε(E) is the field-dependent permittivity, α is the Rayleigh coefficient, εinit is the field-independent initial permittivity, and E is the applied electric field. Significantly, εinit reflects the reversible dielectric response, attributable to lattice deformation and reversible domain wall motion, thus is frequency-dependent. Conversely, the irreversible dielectric response is encapsulated by the coefficient αE. The fitted results align well with the electric-field dependence of dielectric permittivity. The increase in εinit indicates an enhancement in contributions from reversible domain wall motion and intrinsic ionic response, as shown in Fig. 4e. The coefficient α initially increases and then decreases with increasing Sn, suggesting that the irreversible domain activity becomes stronger firstly and then attenuated (See Fig. 4f), regardless of the frequency. As frequency increases, both εinit and α decrease in the ceramics. The reduction in εinit may be ascribed to a weakened reversible domain response, while the decline in α results from deteriorated irreversible domain activity under a higher frequency. Additionally, the intrinsic contributions can be estimated using the following equation:
where Cintri represents the proportion of intrinsic contribution. Notably, the increased Cintri indicates that the intrinsic contribution rises with increasing Sn, as shown in Fig. 4g. In addition, the larger proportion of intrinsic contribution makes a major contribution to the εr, as does d33. Thus, the intrinsic contribution to the piezoelectric property is also enhanced in the ceramics with increasing Sn.
Discussion
In summary, the origin of the ultrahigh piezoelectric performance has been revealed in Sn-doped BT-based ceramics with the nonstoichiometric Sn strategy. To understand how Sn dopant contributes to enhancing piezoelectric properties, the Sn-induced intrinsic and extrinsic contribution is systematically investigated in BSTS0.08+x ceramics. It is found that the common supports from the intrinsic and extrinsic contributions facilitate the ultrahigh piezoelectricity in Sn-doped BT-based ceramics. The intrinsic contribution pertains to the linear piezoelectric effect associated with lattice distortion. The enhancement of polarization arises from lattice distortions and increased space for Ti-O bonds, induced by a small amount of Sn doping, which intrinsically contributes to enhancing the piezoelectric properties. Conversely, the extrinsic contribution reflects the piezoelectric response resulting from the movement of interfaces within the piezoelectrics, particularly phase boundary and domain wall. With the Sn doping, the evolution of phase structure from O-T to R-O-T phases coexistence and the miniaturized domains result in a nearly vanishing polarization anisotropy and thus low domain wall energy facilitating easy polarization rotation and domain wall motion, which finally leads to strong piezoelectricity. This work provides a fundamental understanding of the role of Sn dopant, offering valuable insights for designing doping processes and developing high-performance piezoelectric materials.
Methods
Sample preparation
Ba0.86Sr0.14Ti0.92Sn0.08+x (x = −0.02, 0, and 0.02, abbreviated as BSTS0.08+x) ceramics were prepared by conventional solid-state method. Raw materials BaCO3 (99.0%), SrCO3 (99.0%), SnO2 (99.5%), and Ti-O2 (98.0%) produced by Sinopharm Chemical Reagent Co., Ltd., were weighed and ball milled for 24 hours with both ZrO2 ball media and alcohol. The powder mixture was calcined at 1200 °C for 2 hours. Subsequently, the powders were pressed into pellets with the addition of 8 wt% polyvinyl alcohol (PVA). The PVA was then thermally decomposed at 850 °C, followed by sintering at approximately 1400 °C for 3 hours. The resulting samples were coated with silver paste to form electrodes. Finally, the samples were poled under a direct current field of approximately 30 kV/cm for 30 minutes.
Structure characterizations
The XRD patterns were gained by X-ray diffraction (XRD) machine with Cu kα radiation (Bruker D8 Advanced XRD, Bruker AXS Inc., Madison, WI). Raman spectra were conducted by a Horiba Aramis Raman spectrometer (Horiba Scientific) with excitation sources of 532 nm. X-ray photoelectron spectroscopy (XPS) was obtained by X-ray photoelectron microprobe (XPS, K-Alpha+, Thermo Fisher Scientific, USA). The element distribution was analyzed by an electron probe microanalyzer (EPMA, JEOL JXA-8530F) with a wavelength dispersive spectrometer, and the probe size used is ~40–100 nm. Piezoresponse force microscopy (PFM) was conducted by a commercial microscope (MFP-3D, Asylum Research, Goleta, CA), applied to a conductive Pt-Ir-coated cantilever PPP-NCHPt (Nanosensors, Switzerland) with a bias voltage of 1 V. The local piezoresponse loops were obtained by the switching spectroscopy PFM mode with a triangular-wave voltage of 10 V. The electron-transparency sample for transmission electron microscopy (TEM) measurement was obtained by argon-ion beam milling system (Gatan PIPS 695, Gatan Inc., USA) under 0.1–6 kV. Then, the TEM measurement was conducted by a high-resolution TEM microscope (2100 F, JEOL, Japan) under 200 kV. The ceramic surface morphology was observed via a scanning electron microscopy (SEM) ( JSM-6460LV, JEOL, Tokyo, Japan).
Electrical properties measurements
The temperature-dependent dielectric properties at −60 ~ 150 °C were measured by an LCR analyzer (E4980A, Keysight, U.S.A.) at the frequency of 1, 10, and 100 kHz. The d33 was measured with a commercial Berlincourt-type d33 meter (ZJ-3A, China) for the poled samples. The ferroelectric hysteresis (P-E) loops and strain curves (S-E) were measured by a ferroelectric tester (aixACC TF Analyzer 2000 E, Germany) under a frequency of 1 Hz. Subcoercive measurements of the dielectric permittivity on unpoled samples were carried out via an impedance analyzer (Alpha-Analyzer, Novocontrol, Germany) interfaced with a high-voltage unit (HVB 300, Novocontrol, Germany). The complex impedance spectra were measured by a Novocontrol alpha-A impedance analyzer (Novocontrol Technologies GmbH & Co. KG, Montabaur, Germany) from 1 Hz to 3 MHz in the temperature range of 260–350 °C.
DFT calculation
The bulk structures of Ba(Ti1-x, Snx)O3 including the BaTiO3, Ba(Ti0.875, Sn0.125)O3, Ba(Ti0.75, Sn0.25)O3, and BaSnO3 with cubic and tetragonal phases were studied by the first-principles density functional theory (DFT) calculations. The generalized gradient approximation (GGA) with the Perdew-Burke-Ernzerhofer (PBE) exchange-correlation function was employed in the calculations by using the Vienna Ab initio Simulation Package (VASP) with energy cutoff 500 eV47,48,49,50. The convergence criterion of energy and Helmann-Feynman force is set at 10−5 eV and 10−3 eV/Å. The k-points mesh for single-cell structures (BaTiO3 and BaSnO3) is 13*13*13, while k-points mesh in the remaining supercell structures (Ba(Ti0.875, Sn0.125)O3 and Ba(Ti0.75, Sn0.25)O3) is 6*6*6. The Γ4- mode analysis is achieved with the support of ISODISTORT developed by Brigham Young University51,52. The visualization of calculation results is presented by VESTA53 (See Fig. S9) and Paraview54 (See Fig. S10). The Born Effective Charge (BEC) is calculated by using density functional perturbation theory (DFPT). The calculation of the Bader charge is implemented based on Bader Charge Analysis code55.
Data availability
The source data generated in this study are provided in the Source Data file. More relevant data sets generated during and/or analyzed during the current study are available from the first authors and corresponding authors on reasonable request. Source data are provided with this paper.
References
Jaffe, B., Cook, W. R. & Jaffe, H. Piezoelectric ceramics. (Academic Press, London, 1971).
Zhang, S. J. et al. Advantages and challenges of relaxor-PbTiO3 ferroelectric crystals for electroacoustic transducers–A review. Prog. Mater. Sci. 68, 1–66 (2015).
Zheng, T., Wu, J., Xiao, D. & Zhu, J. Recent development in lead free perovskite piezoelectric bulk materials. Prog. Mater. Sci. 98, 552–624 (2018).
Shrout, T. R. & Zhang, S. J. Lead-free piezoelectric ceramics: Alternatives for PZT? J. Electroceram. 19, 113–126 (2007).
Rödel, J. & Li, J. F. Lead-free piezoceramics: status and perspectives. MRS. Bull. 43, 576–580 (2018).
Hong, C. H. et al. Lead-free piezoceramics–where to move on? J. Materiomics 2, 1–24 (2016).
Hao, J. G., Li, W., Zhai, J. & Chen, H. Progress in high-strain perovskite piezoelectric ceramics. Mater. Sci. Eng. Rep. 135, 1–57 (2019).
Jo, W. et al. Giant electric-field-induced strains in lead-free ceramics for actuator applications - status and perspective. J. Electroceram. 29, 71–93 (2012).
Acosta, M. et al. BaTiO3-based piezoelectrics: fundamentals, current status, and perspectives. Appl. Phys. Rev 4, 041305 (2017).
Liu, W. F. & Ren, X. B. Large piezoelectric effect in Pb-free ceramics. Phys. Rev. Lett. 103, 257602 (2009).
Bell, A. J. & Deubzer, O. Lead-free piezoelectrics—the environmental and regulatory issues. MRS Bull 43, 581–587 (2018).
Zhao, C., Huang, Y. & Wu, J. Multifunctional barium titanate ceramics via chemical modification tuning phase structure. InfoMat. 2, 1163–1190 (2020).
Gao, J. et al. Enhancing dielectric permittivity for energy-storage devices through tricritical phenomenon. Sci. Rep. 7, 40916 (2017).
Shi, T., Xie, L., Gu, L. & Zhu, J. Why Sn doping significantly enhances the dielectric properties of Ba(Ti1-xSnx)O3. Sci. Rep. 5, 8606 (2015).
Kalyani, A. K., Brajesh, K., Senyshyn, A. & Ranjan, R. Orthorhombic-tetragonal phase coexistence and enhanced piezo-response at room temperature in Zr, Sn, and Hf modified BaTiO3. Appl. Phys. Lett 104, 252906 (2014).
Gao, J. et al. Understanding the mechanism of large dielectric response in Pb-free (1-x)Ba(Zr0.2Ti0.8)O3-x(Ba0.7Ca0.3)TiO3 ferroelectric ceramics. Acta Mater. 125, 177–186 (2017).
Lei, C., Bokov, A. A. & Ye, Z. G. Ferroelectric to relaxor crossover and dielectric phase diagram in the BaTiO3-BaSnO3 system. J. Appl. Phys 101, 084105 (2007).
Zhao, C. et al. Practical high piezoelectricity in barium titanate ceramics utilizing multiphase convergence with broad structural flexibility. J. Am. Chem. Soc. 140, 15252–15260 (2018).
Chen, K. et al. Improve piezoelectricity in BaTiO3-based ceramics with large electrostriction coefficient. J. Mater. Sci: Mater. Electron 31, 12292–12300 (2020).
Chen, K. et al. Large piezoelectric performance in zirconium doped Ba0.86Sr0.14TiO3 lead-free ceramics through utilizing multiphase coexistence. J. Mater. Sci: Mater. Electron. 30, 18336–18341 (2019).
Zheng, H. et al. Synergic and competitive effect of A-site substitution on structure and electric property in BaTiO3-based ceramics. J. Alloys Compd. 937, 168352 (2023).
Cohen, R. E. Origin of ferroelectricity in perovskite oxides. Nature 358, 136–138 (1992).
Goldschmidt, V. M. Die gesetze der krystallochemie. Naturwissenschaften 14, 477–485 (1926).
Wu, B. et al. Insights into the correlation between ionic characteristics and microstructure and multiferroic properties in KNN-based ceramics with BiMO3 modification. J. Alloys Compd. 966, 171568 (2023).
Pan, W. et al. Defect chemistry of A site nonstoichiometry and the resulting dielectric behaviors in SrxTi0.985(Nb2/3Zn1/3)0.015O3 ceramics. J. Am. Ceram. Soc. 103, 6298–6307 (2020).
Horchidan, N. et al. Multiscale study of ferroelectric-relaxor crossover in BaSnxTi1−xO3 ceramics. J. Eur. Ceram. Soc. 34, 3661–3674 (2014).
Slodczyk, A. & Colomban, P. Probing the nanodomain origin and phase transition mechanisms in (un)poled PMN-PT single crystals and textured ceramics. Materials 3, 5007–5028 (2010).
Veerapandiyan, V. K. et al. B-site vacancy induced Raman scattering in BaTiO3-based ferroelectric ceramics. J. Eur. Ceram. Soc. 40, 4684–4688 (2020).
Tougaard, S. Practical guide to the use of backgrounds in quantitative XPS. J. Vac. Sci. Technol. A 39, 011201 (2020).
Shard, A. G. Practical guides for x-ray photoelectron spectroscopy: Quantitative XPS. J. Vac. Sci. Technol. A 38, 041201 (2020).
Tougaard, S. Formalism for quantitative surface analysis by electron spectroscopy. J. Vac. Sci. Technol. A 8, 2197–2203 (1990).
Zhang, K. et al. The self-compensation mechanism in barium titanate ceramics with colossal permittivity. J. Alloys Compd. 851, 156856 (2021).
Feng, Y. et al. Defects and Aliovalent Doping Engineering in Electroceramics. Chem. Rev. 120, 1710–1787 (2020).
Qiao, X. S. et al. Microstructure and electrical properties of nonstoichiometric 0.94 (Na0.5Bi0.5+x)TiO3-0.06BaTiO3 lead‐free ceramics. J. Am. Ceram. Soc. 99, 198–205 (2016).
Wang, F. H. Evolution of microstructure and properties in BT-based lead-free magnetoelectric composite. Ceram. Int. 48, 27472–27478 (2022).
Wu, Y., Miao, J., Liu, Z. & Li, Y. Colossal permittivity and dielectric relaxations in BaTi0.99(Nb0.5Ga0.5)0.02O3 ceramics. Ceram. Int. 41, 846–850 (2015).
Kang, B. S., Choi, S. K. & Park, C. H. Diffuse dielectric anomaly in perovskite-type ferroelectric oxides in the temperature range of 400-700 °C. J. Appl. Phys 94, 1904–1911 (2003).
Wu, B. et al. Evolution of multilevel structures and electrical properties in potassium-sodium niobate-based lead free piezoceramics by anionic fluorine engineering. J. Alloy. Compd. 918, 165604 (2022).
Balke, N., Lupascu, D. C., Granzow, T. & Rödel, J. Fatigue of lead zirconate titanate ceramics. i: unipolar and DC loading. J. Am. Ceram. Soc. 90, 1081–1087 (2007).
Zhang, M. H. et al. Origin of high electromechanical properties in (K, Na)NbO3-based lead-free piezoelectrics modified with BaZrO3. Phys. Rev. Mater. 4, 064407 (2020).
Wu, B. et al. Giant piezoelectricity and high Curie temperature in nanostructured alkali niobate lead-free piezoceramics through phase coexistence. J. Am. Chem. Soc. 138, 15459–15464 (2016).
Zhang, M. H. et al. Deciphering the phase transition-induced ultrahigh piezoresponse in (K, Na)NbO3-based piezoceramics. Nat. Commun. 13, 3434 (2022).
Wu, B. et al. Contribution of irreversible non-180° domain to performance for multiphase coexisted potassium sodium niobate ceramics. Nat. Commun. 15, 2408 (2024).
Lin, D. B. et al. Domain size engineering in tetragonal Pb(In1/2Nb1/2)O3-Pb(Mg1/3Nb2/3)O3-PbTiO3 crystals. J. Appl. Phys. 110, 084110 (2011).
Lu, N. & Zhu, J. Electron low energy-loss functions of Pb (Mg1/3Nb2/3)O3: Theory and experiment. J. Appl. Phys. 104, 034109 (2008).
Choi, K. J. et al. Enhancement of ferroelectricity in strained BaTiO3 thin films. Science 306, 1005–1009 (2004).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Kresse, G. Efficient iterative schemes for Ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558 (1993).
Campbell, B. J., Stokes, H. T., Tanner, D. E. & Hatch, D. M. ISODISPLACE: a web-based tool for exploring structural distortions. J. Appl. Crystallogr. 39, 607 (2006).
Stokes, H. T., Orden, S. V. & Campbell, B. J. ISOSUBGROUP: an internet tool for generating isotropy subgroups of crystallographic space groups. J. Appl. Crystallogr. 49, 1849 (2016).
Momma, K. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272 (2011).
Ayachit U. The paraview guide: a parallel visualization application. Kitware, Inc. (2015).
Yu, M. & Trinkle, D. R. Accurate and efficient algorithm for Bader charge integration. J. Chem. Phys 134, 064111 (2011).
Acknowledgements
K. Wang acknowledges the support of the National Nature Science Foundation of China (Nos. 52325204, 52032005). H. -C. Thong acknowledges the support of Beijing Natural Science Foundation-International Scientists Project (No. IS24026). H. Tao acknowledges the support of the National Natural Science Foundation of China (No. 52202149), the Foundation of Sichuan Province Science and Technology Support Program (No. 2023NSFSC0968), and the Fundamental Research Funds for the Central Universities, Southwest Minzu University (No. ZYN2023110). C. Zhao acknowledges the support of the National Natural Science Foundation of China (No. 12104093). B. Han acknowledges the support of the National Natural Science Foundation of China (No. 51972005, U21A2055).
Author information
Authors and Affiliations
Contributions
B.W., C.Z., F.Z.Y., and B.H. conceived the idea of this work. H.Z. and H.T. prepared the ceramics. Z.X. and Y.X.L. performed the electrical and structural measurements and analyzed the data with the guidance by J.M. and K.W.Y. Q.W. and H.C.T. performed the DFT Calculation. H.T., C.Z., B.H., and K.W. provided financial and technical support for this work. Z.H., Z.X., C.F.W., and N.L. help to revise the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Hua Hao and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, B., Zheng, H., Wu, YQ. et al. Origin of ultrahigh-performance barium titanate-based piezoelectrics: Stannum-induced intrinsic and extrinsic contributions. Nat Commun 15, 7700 (2024). https://doi.org/10.1038/s41467-024-52031-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-52031-z
- Springer Nature Limited