Abstract
The megadiverse plant family Asteraceae forms an iconic component of island floras including many spectacular radiations, but a global picture of its insular diversity is lacking. Here, we uncover the global biogeographical and evolutionary patterns of Asteraceae on islands to reveal the magnitude and potential causes of their evolutionary success. We compile a global checklist of Asteraceae species native and endemic to islands and combine it with macroecological analyses and a phylogenetic review of island radiations. Asteraceae have a global distribution on islands, comprising approximately 6,000 native island species, with 58% endemics. While diversity of the family on islands is lower than expected given its overall diversity, Asteraceae are the most diverse family on oceanic islands, suggesting an exceptional ability to thrive in isolation. In agreement with island biogeography predictions, native Asteraceae diversity increases with area and decreases with isolation, while endemism increases with both. We identify 39 confirmed island radiations and 69 putative radiations, exceeding numbers for other iconic insular groups. Our results reveal Asteraceae offer immense potential for research in ecology and evolution, given their close tracking of island biogeography expectations, large number of both species and radiations, cosmopolitan distribution, and numerous undiscovered radiations.
Similar content being viewed by others
Introduction
The top ten most diverse plant families make up 43% of angiosperm species1. Understanding the distribution and drivers of diversity of these large families is thus a crucial step towards explaining the success of flowering plants in general. Key biogeographical settings for exploring the patterns and processes that shape angiosperm diversity are islands. Due to their distinct boundaries, global distribution, and replication, island systems have played a crucial role in the development of key evolutionary and ecological theories2,3,4,5,6,7,8. The geographic isolation and unique habitats found on islands have given rise to remarkable angiosperm biodiversity that is often characterized by high levels of endemism9,10, adaptive radiations11,12, paleoendemism13,14, and repeated evolution of convergent traits15,16.
While islands are valuable natural laboratories for studying plant diversity, global-scale data on the distribution of major plant families on islands are only starting to emerge. Recent global studies have explored biodiversity patterns for a few major families and lineages on islands17,18, factors impacting the assembly of island floras19,20,21,22, and traits associated with insular diversity23,24,25,26. These studies reveal how links between island features (e.g., area, isolation, age, and climate), functional traits (e.g., insular woodiness), and biogeographical rates (e.g., colonization, speciation, and extinction) are important determinants of the number of native and endemic species of flowering plants on islands, whilst suggesting that evolutionary success on islands may not necessarily mirror that found on continents27.
Out of all plant families, arguably the one most often associated with evolutionary success on islands is the most diverse family of all—Asteraceae. Commonly known as the daisy or sunflower family, Asteraceae (Compositae) boasts the greatest species number of any plant family in the world, with an estimated 32,000–34,000 species1,28,29. Species of this family are native to every continent except Antarctica and are found in a wide range of habitats, but are most abundant in dry and semi-arid habitats and in Mediterranean-type ecosystems, deserts, grasslands, and mountains30. Members of the family show great variation in growth habits and range from small annual herbs to woody perennial shrubs, lianas, and trees. Additionally, some species exhibit specialized habits such as cushion forms, succulents, and even, in rare cases, are epiphytic and aquatic plants.
On islands, Asteraceae are thought to be remarkably diverse, and often form an iconic component of insular floras of both continental and oceanic origin. For instance, it is the most species-rich family on the remote Juan Fernández Archipelago31 with 30 native species and four genera endemic to the islands, and is among the top five most diverse families on the large continental island of Madagascar32. Additionally, the family has high levels of endemism on oceanic islands: a study by Lenzner et al.33 compiled diversity data on major plant families across 14 oceanic archipelagos and found that Asteraceae had the highest number of single-island endemics for the oceanic islands considered in the study. Their success in dispersal, establishment, and diversification on islands has been suggested to result from a combination of intrinsic factors3,34,35: Asteraceae possess unique fruit morphology that aids in long-distance dispersal36,37; their head-like inflorescence (capitulum) often attracts generalist pollinators; and they are capable of several breeding systems that may favor establishment on islands. Many island species are self-compatible23, while species in several island lineages are functionally self-incompatible with the capacity to self-seed and a genetic system (i.e., sporophytic self-incompatibility) that aids both in the establishment of small populations and the retention of genetic diversity after arrival38.
In addition to a high native and endemic species richness on islands, Asteraceae are known for their presumed propensity to radiate (that is, to undergo cladogenesis in situ on islands at relatively fast rates). Two recent studies, one reviewing adaptive radiations across flowering plants39 and another focused on adaptive radiations on oceanic islands across all taxonomic groups40, both found Asteraceae to be overrepresented in terms of adaptive radiations compared to other clades. Indeed, the family provides numerous examples of spectacular island radiations: Scalesia on the Galápagos Islands41, the woody Sonchus alliance on the Canary Islands42, Dendroseris on the Juan Fernández Islands43,44. One of the textbook examples of adaptive radiation on islands is the Asteraceae silversword alliance of Hawai‘i, a clade of 33 species in three endemic genera (Argyroxiphium, Dubautia, Wilkesia), which evolved from a common ancestor that colonized Hawai‘i by a long-distance dispersal event from North America around 5 million years ago (Mya), and which exhibit high diversity in morphology and ecological adaptation45,46,47. Another notable example is the Hawaiian Bidens. The monophyletic 20 species of Bidens endemic to Hawai‘i originated from a single colonization event c. 1.8 Mya, having thereafter radiated across the archipelago, occupying a wide variety of different habitats including sand dunes, lava fields, rainforests, and wetland bogs, and have the highest rates of speciation per unit area documented for any island plant radiation to date48,49.
An increasing number of phylogenetic studies focusing on selected island clades of Asteraceae from specific islands or archipelagos41,47,50,51,52 are providing insight into the potential drivers of diversification in those Asteraceae groups. One hypothesis is that the high diversity of Asteraceae on islands results from a combination of high continental diversity, high rates of long-distance dispersal, and overall high rates of in situ speciation that well exceed extinction rates (consistent with the high net diversification rates observed in continental Asteraceae)53,54,55,56.
While it is assumed from the above examples that Asteraceae are highly diverse on islands and have a propensity to radiate, in fact, a complete global picture of the diversity and distribution of the family is yet to be assembled. Furthermore, an assessment of Asteraceae’s potential to radiate across islands globally is still lacking, because previous studies focused solely on confirmed adaptive radiations and/or on oceanic islands, and thus the magnitude of island radiations within the family is unknown.
In this work, we compile a global checklist of island Asteraceae and use this to answer four key questions: (1) How does the island species richness of Asteraceae compare with that of other flowering plant families? (2) How is island Asteraceae diversity distributed across space and major clades of the family? (3) What are the environmental and biogeographical drivers of native and endemic insular diversity on islands? (4) How many island radiations have occurred within Asteraceae and are there commonalities between radiations? We show that Asteraceae have a truly global distribution on islands, with 6135 native island species, 58% of which are endemic. Our findings reveal that while the diversity of the family on islands is lower than expected given their overall diversity, Asteraceae are the most diverse family on oceanic islands, suggesting an exceptional ability to thrive in isolation. Moreover, diversity patterns in Asteraceae follow classic island biogeography theory, with area and isolation being the strongest predictors of species richness and endemism, and the numerous confirmed and understudied radiations highlight the family’s potential as an ideal study system in ecology and evolution.
Results and discussion
Asteraceae are one of the most diverse families on islands
Among angiosperms, Asteraceae are the largest family in the world with 32,000–34,000 species globally. Our comprehensive checklist of insular Asteraceae shows that this family is also remarkably diverse on islands: we found 6135 species of Asteraceae are native to islands, of which 3535 (58%) are endemic to islands globally. On oceanic islands specifically, we found 1833 native Asteraceae species and 955 (52%) endemic species.
As Asteraceae species are generally perceived to be good dispersers and excellent island colonizers3,36, the proportion of island native and endemic species of the total Asteraceae species pool would be expected to be higher in Asteraceae than in other large families, and higher than expected by chance. Surprisingly, our comparison between the diversity of angiosperm families on islands showed that Asteraceae are not the most species-rich family across all islands (Fig. 1 and Supplementary Table 2) and that they are underrepresented in terms of island species given its overall diversity (Fig. 1 and Supplementary Table 3). These results align with a recent study focused on island disharmony in plants22, which found that while Asteraceae are generally underrepresented on islands given their species richness in mainland source pools, the family is nonetheless pervasive on islands. Orchidaceae and Rubiaceae have the highest number of native island species with 11,188 and 6188 species respectively. The high insular diversity of Orchidaceae and Rubiaceae is found disproportionately on large, tropical continental islands and archipelagos (including New Guinea, Borneo, and the Philippines), which are not particularly rich in Asteraceae species. On oceanic islands, Asteraceae are the most diverse family for both the number of native and endemic species. Yet, despite high species richness compared to other families, Asteraceae diversity on oceanic islands is lower than expected given their diversity globally (Fig. 1B).
Species diversity of these families on A all islands and B oceanic islands compared to the null expectation. The left panels rank the ten most diverse angiosperm families on islands for native (blue and purple points) and endemic (green and orange points) species. Families are ranked in descending order by the number of native species. The right panel compares the observed number of native island species per family (points) to the null expectation of island diversity (histogram). Families for which the observed number of species is lower than the null expectation are highlighted with a red point, and those above the null expectation in black. Source data are provided in the Source Data file. The global diversity of each family is listed in Supplementary Table 2.
Island species account for 18% of the total species diversity of Asteraceae (Supplementary Table 2). Using a binomial test we found that the observed number of Asteraceae species native to islands is significantly different than expected based on the proportion of Asteraceae to angiosperms globally (10%) and that the island proportion (6%) is significantly lower than expected under a null model (Supplementary Table 3 and Fig. 1). Additionally, a comparison of the observed number of island Asteraceae species to the island community simulations confirms that the observed number of island species is lower than the null expectation across all islands and oceanic islands (Fig. 1).
Clade age could be a factor contributing to the differences in island diversity between plant families. The diversity of the mainland species pool of each family is influenced by family age and global diversification rates. Notably, among the ten most diverse families on islands, Asteraceae are the youngest family (Supplementary Table 2), which could account for their underrepresentation on islands, as younger families may have been less diverse with smaller mainland pools at the time of emergence of the islands in our dataset, thus giving them a colonization “disadvantage” earlier on. However, most of the islands in our dataset are much younger than the age of the families (Supplementary Fig. 1) suggesting that the primary biogeographic causes of the underrepresentation on islands are to be found in lower colonization and/or diversification rates since island origin. As more phylogenetic data of island species becomes available, models of island biogeography, such as the Dynamic Assembly of Islands through Speciation, Immigration and Extinction (DAISIE) model57,58, can be used to estimate rates of colonization, speciation, and extinction for entire island communities of each family over evolutionary timescales.
Asteraceae have a global distribution across islands
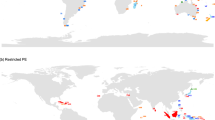
Native species of Asteraceae have a truly global distribution across the world’s islands (Fig. 2). In our global checklists, Asteraceae occur natively on 791 islands including 308 oceanic islands, and across 146 archipelagos. Their distribution reaches north to the Svalbard Islands and Greenland (80°N and 75°N) and south to Macquarie Island and Heard Island (55°S and 53°S). Insular diversity ranged from one (minimum inclusion criterion) to 550 native species, with 29 islands (4%) harboring more than 100 native species and 155 islands (20%) harboring only one native species (our dataset only includes islands with at least one native Asteraceae species). Across all island types, 128 islands (16%) have at least one endemic species.
A Global distribution of Asteraceae across all island types. The shape of the marker represents the island type (i.e., continental or oceanic), the size represents the number of species, and the color indicates the number of endemics, with gray meaning no island endemics. B Global distribution of Asteraceae on archipelagos. Source data are provided in Supplementary Data 3.
While its distribution is global, the diversity of the family is not evenly distributed geographically, and several island regions are notable hotspots of diversity. Madagascar is the most diverse island overall for both native (550) and endemic (487) species. The Caribbean, in particular the Greater Antilles with 671 native and 430 endemic species, is another major center of island Asteraceae diversity. At the island level, three large islands (Cuba, Hispaniola, Jamaica) are all in the top ten most diverse islands globally for number of endemic species (196, 145, and 58). This pattern of the Caribbean as an important area of endemism for the family further supports a review by ref. 59, who found that the region has the highest number of endemic genera in Asteraceae globally. Across oceanic islands, Macaronesia, the Hawaiian Islands, and the Mascarenes are hotspots of island diversity. The Canary Islands is the most diverse oceanic archipelago with 299 native species, and seven of the ten most diverse oceanic islands for native species are islands in the Canaries, with Tenerife being the most species-rich (159 species). The Hawaiian Islands are the second most diverse oceanic archipelago with 102 native and 95 endemic species, and have a remarkably high proportion of endemism (93%), followed by the Mascarenes with 79 native and 64 endemic species.
In comparing hotspot regions, the British Isles (850 native species and 368 endemic species) and Iceland (334 native species and 261 endemic species) stand out as diversity anomalies. While these two regions appear as hotspots of island diversity, the majority of species in these two island regions are apomictic60. Apomixis, a mode of asexual reproduction in which seeds are produced without fertilization, is a poorly understood trait in Asteraceae61 and one that challenges taxonomic species concepts and delimitation62. To investigate the impact of apomictic species on our results, we performed a sensitivity analysis with apomictic genera removed (see Supplementary Fig. 5), which revealed minor changes to the ranking of top island hotspots, but no effect on our findings otherwise (including the models).
The diversity of island species is also unevenly distributed across the major clades and taxonomic tribes of the family (Fig. 3). The tribe with the highest number of native island species is Cichorieae (1660 spp.); while this tribe is an important component of island floras (e.g., Tolpis and the woody Sonchus alliance in Macaronesia, Dendroseris in the Juan Fernández Islands), its overall diversity is inflated due to the high number of apomictic species, well-known in this tribe (e.g., Hieracium on Iceland, Taraxacum on the British Isles). Aside from Cichorieae, the three most diverse tribes for both native and endemic island species are Astereae (793 native island species, 465 endemic species), Senecioneae (653, 447), and Gnaphalieae (589, 339). Together, these four widespread tribes make up nearly 60% of all native insular Asteraceae species (Supplementary Table 4 and Supplementary Fig. 3). While these tribes are also some of the largest tribes in the family, when we compare observed island diversity to expected diversity given the overall size of the tribe (Supplementary Table 5), we find that island species are overrepresented in Cichorieae, Astereae, and Gnaphalieae and within the expected range for Senecioneae. The two tribes with the highest proportion of native island species compared to the total diversity are Feddeeae (100%) and Distephaneae (86%). Feddeeae is a monotypic tribe with a single species, Feddea cubensis endemic to Cuba (Supplementary Fig. 3). The Distephanus clade is a group distributed across Africa, Madagascar, and the Mascarenes and has a notable overrepresentation of island species relative to overall diversity (36 island species, 43 total species) (Supplementary Table 5).
A Time-calibrated molecular phylogeny of the tribes and major clades within Asteraceae from ref. 54. Tribes are colored by subfamily classification. B The number of species native to islands (dark bar) compared to the overall number of species globally (light bar) in each tribe. The percentage of native island species to total species globally is specified next to each tribe. Illustrations highlight clades with high island diversity: (1) Argyroxiphium sandwicense endemic to Hawai‘i, Madieae; (2) Commidendrum rugosum endemic to Saint Helena, Astereae; (3) Abrotanella inconspicua endemic to New Zealand, Senecioneae; (4) Distephanus populifolius endemic to Mauritius, Distephaneae; (5) Anastraphia ilicifolia endemic to Cuba, Gochnatiaeae. Plant illustrations by Lizzie Roeble, and originally featured in the CAPITULUM. The Asteraceae phylogeny is adapted from Mandel, J. R. et al. A fully resolved backbone phylogeny reveals numerous dispersals and explosive diversifications throughout the history of Asteraceae. Proc. Natl. Acad. Sci. USA 116, 14083–14088 (2019). The phylogeny and plant illustrations are released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license (https://creativecommons.org/licenses/by-nc-nd/4.0/). Source data are provided in the Source Data file.
The intrafamily diversity patterns are influenced by both the global distribution of a tribe and the presence of islands within that range. Asteraceae clades that have an overrepresentation of island species despite limited islands available within their overall range likely have intrinsic traits potentially well-adapted to islands. Additionally, several tribes with high island species richness, such as Gnaphalieae and Senecioneae, are well-known for having widespread “weedy” species characterized by a combination of traits that promote colonization, fast growth, and dominance. Future research on the widespread weedy species that occur natively on islands could provide insight into traits that facilitate successful colonization and establishment in novel habitats on islands.
Drivers of island species richness and proportion endemism
We ran generalized linear mixed models (GLMMs) to explore which island features and environmental variables may be driving Asteraceae native species richness (NSR) and the proportion of single-island endemics (pSIE) across islands. Diversity patterns in Asteraceae follow classic island biogeography theory, with area and isolation (-SLMP) being the strongest predictors of both NSR and pSIE (Fig. 4). Area has a positive association with NSR (β = 0.64, 95% CI 0.56–0.72) and the pSIE (β = 1.55, 95% CI 1.17–1.94) (Supplementary Table 6). This pattern of an increasing number of species with area conforms with both the species-area relationship63 and the Theory of Island Biogeography2 and is well-supported across various island systems in other taxonomic groups57,64,65,66. While isolation is a strong predictor of both NSR and pSIE, it has an inverse relationship on the two measures of diversity, having a negative effect on NSR (β = −0.32, 95% CI −0.45 to −0.19) but a positive effect on pSIE (β = 0.48, 95% CI 0.09–0.88), with more isolated islands having a higher proportion of endemism. The increase in endemism with isolation is also a prediction of island biogeography, as MacArthur and Wilson proposed the existence of a zone of radiation, where diversification should outpace the dispersal-mediated build-up of species on near islands, and islands change from a dispersal- to an evolution-driven system as isolation increases2,57,67,68.
A Maximum likelihood estimates of coefficients with their 95% confidence interval for the global models of native species richness (blue, n = 510 islands) and the proportion of single-island endemics (green, n = 510 islands). The gray vertical line at 0 indicates no effect, and island variables with a positive coefficient estimate indicate an increase in NSR or pSIE, whereas a negative coefficient estimate indicates a decrease in the response variables. B Marginal effects for the island and environmental variables. The black line represents the predicted response under the model (mean values) and the gray band is the 95% confidence interval. The predictor variables for area, isolation (-SLMP), maximum elevation, and temperature seasonality were log-transformed to address skewness. All predictors were standardized (centered and scaled) to enable direct comparison of their effects. The x-axis for these variables reflects the standardized log-transformed scale, except for annual temperature, which is standardized but not log-transformed. Source data are provided in Supplementary Data 3.
Island type (oceanic vs continental), which represents the geological origin of islands and is a proxy for connectivity over time, affects both NSR and pSIE. Oceanic islands have fewer native species (β = −0.38, 95% CI −0.57 to −0.19), and a higher proportion of single-island endemics (β = 1.36, 95% CI 0.48–2.23). Maximum elevation has a positive effect on NSR (β = 0.13, 95% CI 0.05–0.22), with higher islands having more native species. Temperature seasonality is the best climatic predictor for NSR (positive effect), whereas mean annual temperature is the best climatic predictor for pSIE (positive effect). In the subset model that was filtered to oceanic islands and included Age + Age2 as an additional predictor, we did not observe an additional effect of island age on NSR nor pSIE (Supplementary Table 7 and Supplementary Fig. 4).
Both the NSR and pSIE models have substantial predictive power in explaining island Asteraceae diversity (see methods and Supplementary Fig. 2 for model diagnostics). The overall variance (conditional R2) explained in the NSR model is 90% and the variance explained by the fixed effects alone (marginal R2) is 56% (Supplementary Table 6) (Nakagawa R2 69). In a separate model, with data aggregated for each archipelago, without random effects, the marginal R2 was 75.1%, with model coefficients all pointing in the same direction as our original model (Supplementary Table 8), indicating the robustness of our qualitative results to geographical scale. In the pSIE model, the overall variance (conditional R2) explained by the model is 69% and the variance explained by the fixed effects alone (marginal R2) is 40%. Comparing the marginal and conditional R2, we find that the inclusion of the archipelago as a random factor contributes to a large proportion of the variance explained in both the NSR and pSIE models. This is likely due to the nature of the island dataset and the common biogeographic history of the islands belonging to an archipelago that contribute to the conditional variance. There are 49 archipelagos in the dataset that are represented by a single island—often due to limited floristic data available, and in these archipelagos, the models have high predictive power. Additionally, the main model patterns and relationships with the predictors are unaffected when apomictic species are removed (see sensitivity analysis in Supplementary Fig. 5).
Island Asteraceae radiations have occurred nearly everywhere
Apart from passerine birds of the Galápagos and Hawai‘i, the Anolis lizards of the Caribbean, or the lemurs of Madagascar, few groups of organisms evoke evolutionary diversification on islands as much as Asteraceae, with its several “flagship” radiations—most famously the Hawaiian silverswords. However, to date, the geographical extent and number of insular radiations in the family have only been studied for a subset of cases (exclusively adaptive radiations from a subset of oceanic islands). Through a comprehensive literature review of island radiations within Asteraceae, we identified 39 phylogenetically confirmed insular radiations and 69 putative taxonomy-based radiations across continental and oceanic islands, totaling 108 island radiations within the family worldwide. The 39 confirmed radiations range in size from three (the minimum threshold) to 160 species, with an average of 18 species per radiation (median = 11) (Fig. 5 and Supplementary Data 4). New Zealand and surrounding islands are home to the two largest radiations: the Celmisia group with c. 160 species and the Raoulia alliance with 42 species. On oceanic islands, the largest radiations are the Polynesian Bidens, with 42 species distributed across Hawai‘i, Marquesas, Society, and Austral Islands, followed by the Hawaiian Silversword alliance with 33 species and the woody Sonchus alliance with c. 31 species radiating on Macaronesia. The mean crown age of the radiations ranges from 0.4 to 24.18 million years (Myr), but the majority of radiations for which a crown age is available are younger than 5 Myr, confirming that they represent mostly recent diversification events.
A Overview of the number of total, confirmed, and putative insular radiations within Asteraceae. The confirmed radiations have been evidenced by robust phylogenetic work and are represented by the black circles, and the putative radiations have been identified based on taxonomy and the island Asteraceae checklist and are represented by the gray circles (see methods for details on assessment criteria). Illustrations of species within the three largest island radiations. B Map compares the number of radiations between regions. In cases where a radiation is distributed across multiple regions, it is included in the region where the most species diversity is located. Several island regions had no radiations (Mediterranean Islands, Micronesia). C The waffle charts summarize characteristics and traits of the confirmed radiations, where a single radiation is represented by one square. Traits were scored at the radiation level, and if there are multi-states in the radiation it is captured with the ''Mixed” category. Plant illustrations by Lizzie Roeble, originally featured in the CAPITULUM and released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license (https://creativecommons.org/licenses/by-nc-nd/4.0/). Full review of island radiations in Supplementary Data 4.
Asteraceae have radiated across a wide geographic range of islands, with radiations found on large continental islands (e.g., Apodocephala-Lowryanthus on Madagascar) to oceanic archipelagos (e.g., Scalesia on the Galápagos) to archipelagos with mixed geologic origin (e.g., Anastraphia on the Caribbean), and from tropical islands (e.g., Hesperomannia on Hawai‘i) to sub-Antarctic islands (e.g., Pleurophyllum across the Auckland, Campbell, and Antipodes Islands, which is nested in the larger Celmisia group radiation). The majority of confirmed radiations have occurred on oceanic islands (26/39 radiations, Fig. 5), and while our mixed effects models support the strong, positive association of isolation on endemism, this could also be a reflection of previous island research focusing on oceanic systems. While radiations have occurred worldwide, several regions are notably rich in confirmed radiations. At least seven radiations with a total of 302 species have occurred on New Zealand and outlying Subantarctic islands. Macaronesia and the Hawaiian Islands also disproportionately support a high number of Asteraceae radiations, with at least ten radiations of 120 species total on Macaronesia, and six radiations comprising 90 species on Hawai‘i. If we also consider putative radiations, the number of radiation in these two regions increases to 15 and 8, respectively.
Despite the high number of confirmed radiations, it is only when surveying the putative radiations that we begin to see the remarkable degree to which this family has, potentially, speciated across islands. In our review, we identified 69 putative radiations, which range in size from three to 67 species (Supplementary Data 4). In general, many of the putative radiations are found within geologically complex regions or fall within large, taxonomically complex clades. More specifically, several regions have a high number of putative radiations. The Caribbean is a known hotspot of Asteraceae diversity59, and we identified four confirmed radiations in this region that were represented in a well-sampled phylogeny and 26 putative radiations with an overall total of c. 351 species. Madagascar is a hyper-diverse island with high endemism and Asteraceae are one of the five most species-rich plant families composing the island’s flora32; we found two confirmed radiations on Madagascar and identified 16 putative radiations that require future phylogenetic work to investigate and delineate. Additionally, while several genera on Madagascar (i.e., Helichrysum, c. 110 endemic species; Senecio, c. 50 endemic species; Vernonia, c. 70 endemic species) meet our criteria of a putative radiation, they were not included in the putative radiation list because these genera are known to be taxonomically complex (paraphyletic and polyphyletic) and distinguishing the potential radiation from multiple colonization events, even tentatively, is too challenging without a well-sampled phylogeny70,71,72,73.
Basing the assessment of putative radiations on taxonomy alone has the potential to under- and overestimate the number of island radiations. On the one hand, an under-estimate of the number of radiations can occur when numerous island endemics within a single large genus arise from multiple independent colonization events and subsequent radiations. For example, phylogenetic work on Psiadia on the Indian Ocean islands supports two independent radiations on the Mascarenes50. On the other hand, an over-estimation can occur when numerous small island-endemic genera are actually part of one larger island radiation. This can lead to two assessment errors: the small island-endemic genera inflate the putative number of radiations if they meet the threshold criteria of three endemic species or the size of the actual radiation is obscured when the small endemic genera are segregated out based on the taxonomy. For example, the woody Sonchus alliance on Macaronesia comprises six genera, but from a well-resolved phylogeny42 we know these genera all arose from a single colonization event and radiated across Macaronesia. Notwithstanding these considerations, our assessment of putative radiations not only shows the potential magnitude of radiations within the family but also provides direction for future phylogenetic research on island diversification.
The combination of confirmed and putative radiations totals 108 island radiations within the family, indicating that Asteraceae have the remarkable capacity to radiate across a wide diversity of islands, including oceanic islands and continental islands, islands and archipelagos with varying degrees of area and isolation, and across a wide spectrum of island ecosystems and habitat types. How the overall number of island radiations within Asteraceae compares to other flowering plant families still remains unknown because a comparative analysis of all island radiations has not been conducted. However, recent reviews of radiations with different scopes or on wider taxonomic groups shed light on the magnitude of Asteraceae radiations on islands revealed here. In a review that was restricted to adaptive radiations on oceanic islands40, Asteraceae stood out as the family with the highest number of adaptive radiations (finding 19 radiations) compared to all taxonomic groups (arthropods, birds, mollusks, plants, amphibians, and reptiles). Additionally, a comprehensive review of island radiations in birds74, using the same criteria as used here, found 39 island radiations compared to Asteraceae’s 108 radiations (confirmed and putative). Together, these studies indicate Asteraceae may be exceptionally rich in island radiations compared not only to other flowering plant families but also to other broader taxonomic groups. However, some of those groups are much less diverse than Asteraceae (e.g., ~11,000 bird species compared to the c. 34,000 Asteraceae species), so whether the propensity to radiate is also exceptionally higher in Asteraceae remains to be investigated.
Ultimately, future research should aim to move from identifying radiations to understanding the processes underlying plant diversification. To this end, for confirmed radiations, we examined several different characteristics and traits that are often associated with plant diversification on islands (Fig. 5C and Supplementary Data 4). Out of all the traits reviewed, the one trait that reveals a strong link with radiations is woodiness. The majority of confirmed radiations have at least one woody species, which is in agreement with recent research that secondary (insular) woodiness is associated with accelerated diversification rates and may be a key innovation for insular plants24. A diversity of dispersal syndromes—a key trait in determining island colonization—are represented in Asteraceae island radiations, with wind dispersal (anemochory) most common on less isolated archipelagos (e.g., Macaronesia) and bird dispersal (endozoochory and epizoochory) more common on isolated archipelagos (e.g., Polynesian islands). Hybridization and polyploidy are thought to be common features of adaptive radiations and linked to plant diversification on islands8,40,75,76, and we found these two traits are to some extent associated with island radiations in Asteraceae: both hybridization and polyploidy are documented in 40% of the confirmed radiations. While self-compatibility is often cited to be overrepresented in island plants23,77,78, in our review of breeding systems (self-compatible, self-incompatible, or mixed), we found this trait to be surprisingly data deficient, indicating fertile ground for more research.
Our analysis of the global patterns of diversity and distribution of Asteraceae on islands is an essential first step towards unlocking further research on Asteraceae on islands, moving beyond classic well-studied oceanic islands (e.g., Canaries, Hawai‘i) to cover less well-studied but also Asteraceae-rich regions such as the Caribbean, New Guinea, or the Mascarenes. Asteraceae diversity is unevenly distributed both geographically and across major clades in the family. This opens up the question of what intrinsic traits and external abiotic conditions are driving Asteraceae diversity on islands. The fact that Asteraceae follow key theoretical expectations of island biogeography and macroecology, suggests that they are not an outlier governed by their own biogeographical rules, highlighting their value as models for biogeography. In comparison with other groups, the key advantage of Asteraceae may lie in its unusually large sample sizes in terms of species and radiations, which may allow for circumventing a common limitation of studies of insular assemblages that are typically species-poor. Finally, the large number of potentially undiscovered radiations of Asteraceae suggests that many years of exciting discoveries on the evolution of this family lie ahead.
Methods
Data collection
Island Asteraceae checklist
We compiled a global checklist of Asteraceae native and endemic to islands. The foundation of the island Asteraceae checklist was the Global Inventory of Floras and Traits (GIFT) database (version 3.0)79,80. GIFT collates and leverages plant checklists and floras with regional-level data on distribution, environment, and functional traits and has a particular strength in island floras. We started by extracting all Asteraceae checklists from GIFT where there was at least one species native to an island. Species non-native or introduced to each island were excluded. We did not consider islands with zero Asteraceae in the database because many of these may be false negatives, since GIFT relies on published floras disentangling the true absence of Asteraceae on an island from a data gap is challenging. To facilitate comparison across regions and sources, the GIFT database records the original species names and endemicity status from the primary floras and checklists and standardizes the taxonomy against the World Checklist of Vascular Plants (WCVP)1. For the island Asteraceae checklists we carried out additional curation. Because Asteraceae are a taxonomically complex family, we matched WCVP standardized names against the Global Compositae Database (GCD, https://www.compositae.org/gcd)29 and retrieved the name status (accepted, uncertain, unaccepted) and the tribe and subfamily classification. We further updated the GCD taxonomy to the latest classification outlined in ref. 81 based on the family-level phylogeny in ref. 54.
The final dataset is a global checklist of Asteraceae native to islands and is composed of 915 island checklists (Supplementary Data 1) and supported by 240 primary sources (Supplementary Data 2). The global checklist of insular Asteraceae is structured by island geographic units. For each island in the dataset, we have a checklist of Asteraceae species, name standardization (original name, WCVP name, and GCD name status), reference to the primary source, intrafamily taxonomic classification, the floristic status of the species (native, endemic, non-endemic) to that geographic unit, distribution, and conservation status.
Island features and environmental variables
For each island in the global checklist, we gathered abiotic data on island features and climatic variables known to be important predictors of global diversity on islands2,5,19. Environmental data were available from GIFT, which includes information on abiotic variables for each island in the dataset. We extracted the following variables: latitude and longitude, area (km2), distance to nearest mainland (distance, km), surrounding landmass proportion (SLMP, sum of the proportions of landmass within 100, 1000, and 10,000 km buffer distances)82, last glacial maximum mainland connection (GMMC), last glacial maximum area (LGM area), island age (Mya), mean and maximum elevation (m), terrain ruggedness index (TRI, m), botanical continent (level 1, standardized biogeographic scheme for recording plant distributions defined by the biodiversity information standards (TDWG)), and biome (Ecoregions)83. We classified islands into two physical types based on past connectivity to the mainland: “continental” islands, those located on the continental shelf or continental fragments and previously connected to the mainland, and ”oceanic” islands, built mainly by volcanic activity or sea-floor uplift or atolls and never connected to another landmass. This classification was initially based on the geology category in GIFT, but we manually adapted and assessed it for each island/archipelago. We also included a “mixed” category, for archipelagos composed of a mixture of continental and oceanic islands. We aggregated islands into “archipelago grouping”, a refined and cleaned archipelago assessment based on the GIFT archipelago levels (arch_lvl_1, arch_lvl_2, arch_lvl_3) to capture shared biogeographic and geologic history. For example, all the islands in the Caribbean are grouped together in GIFT under the archipelago classification of the West Indies (GIFT arch_lvl_1), and for this study, we refined the West Indies archipelagos classification to include the Greater Antilles, Lesser Antilles, and the Bahamas as separate archipelagos. All cases in which the archipelago grouping differs from the one in GIFT are highlighted in the data. Additionally, we collected data on four climatic variables (CHELSA 2.1)84 for each island: annual mean temperature (°C), mean annual precipitation (kg m−2), temperature seasonality (°C/100), and precipitation seasonality (kg m−2). As a result, our global island Asteraceae checklist includes Asteraceae diversity data and associated island spatial and environmental data (Supplementary Data 1).
Comparison of island diversity among flowering plant families
To contextualize the insular diversity of Asteraceae, we compared it with other flowering plant families by gathering island diversity data for all angiosperm families that natively occur on islands following a similar procedure. From GIFT, we extracted every island checklist with at least one native angiosperm species. Then for each family, we calculated the total number of species native to islands and the total number of species endemic to islands. We calculated insular diversity for each family across both (a) all island types (continental, oceanic, and mixed) and (b) only oceanic islands. This provided us with a global assessment of island diversity across flowering plant families, illustrating which families have the greatest diversity of native and endemic species on islands. For each of the top ten most diverse families, we also compiled their clade ages by extracting the stem age from the angiosperm dated phylogeny (relaxed calibration and complete fossil dataset) constructed in ref. 85.
To determine whether island diversity was higher or lower than expected given the overall number of species within each family, we ran binomial tests and simulated island communities. For each angiosperm family, we performed a binomial test to compare the proportion of island species to the proportion of total species of that family to angiosperms globally. The binomial test was conducted using the binom.test() function in R, where “x” represents the number of native island species (i.e., number of successes), “n” represents the total number of angiosperm species native to islands (i.e., number of trials), and “p” represents the proportion of the family to angiosperms globally (i.e., probability of success). The number of species within each family and the total number of angiosperm species globally (333,799) were calculated with the World Checklist of Vascular Plants (WCVP)1 and the number of island-native angiosperm species (99,659) and oceanic-island native species (23,853) were calculated with GIFT. With the binomial test, the null hypothesis is that the observed proportion of a family on islands is equal to its frequency globally (p), and the alternative hypothesis is that the observed proportion on islands is not equal to this global frequency.
Additionally, for visualization purposes, we ran simulations to estimate the null expectation of island diversity and compare it to the observed diversity for the ten most diverse families on both all island types and oceanic islands. For the top ten families, we created a global pool that represents the total number of species in each family overall. We randomly sampled from the global pool to create island communities with the same total number of species as the actual number of native island species overall (10,000 iterations). This gives a null distribution of the island diversity for each family given the overall diversity of the family. We then compared the observed island diversity to the null distribution.
Modeling the biogeographical drivers of island diversity
We used generalized linear mixed models (GLMMs) to understand which island features and environmental variables are linked to Asteraceae (1) native species richness (NSR) and (2) proportion of single-island endemics (pSIE) across islands. Prior to modeling, we carried out a thorough exploration of the data following a protocol described in ref. 86. This included inspection and checks for potential outliers, distribution of response variables, zero inflation, collinearity among response variables, pair-wise relationships between response and predictor, and non-independence of the response variable. Several predictor variables showed high collinearity, in particular, variables found to be correlated to isolation (distance, SLMP, GMMC, LGM area, latitude) and topography (mean elevation, maximum elevation, TRI). Hence, we dropped correlated variables to retain one predictor for isolation (SLMP) and one for topography (maximum elevation). Because several predictor variables were skewed, we log-transformed area, SLMP, maximum elevation, mean annual precipitation, temperature seasonality, and precipitation seasonality. All continuous predictor variables were centered and scaled. Additionally, we multiplied SLMP (surrounding landmass proportion) by −1 to convert this variable to a more intuitive proxy for isolation; with this inverse transformation of SLMP, a higher -SLMP refers to a more isolated island. We removed islands smaller than 1 km2 because diversity on these islets is influenced by different processes (i.e., the small-island effect87,88). The final dataset included 510 islands, 272 oceanic and 238 continental islands (Supplementary Data 3).
We employed AIC-based model selection independently for the (1) NSR and (2) pSIE models, choosing the best-supported global model for each from a set of candidate models (19 for NSR and 15 for pSIE) (see Supplementary Table 1). The models for NSR and pSIE are independent, with potentially different environmental variables explaining variation in each of the two measurements of diversity best. In line with the current literature recommendations, we fit the NSR models with a negative binomial and pSIE models with a beta-binomial error distribution89.
In our global model for NSR, we fit a negative-binomial GLMM to predict total native species with area, isolation (-SLMP), island type (categorical with two levels: oceanic and continental), max elevation, and temperature seasonality, with archipelago included as a random effect. In our global model for pSIE, we fit a GLMM using a beta-binomial and native species richness used as weights to predict the pSIE with area, isolation (-SLMP), island type (categorical with two levels: oceanic and continental), max elevation, and mean annual temperature, with archipelago included as a random effect. All models were fit using the glmmTMB package in R90.
Island age is an important variable in island biogeography correlated to species richness5; however, island age is challenging to accurately estimate91,92 and we do not have full coverage of age estimates for all islands in our dataset. Therefore, we ran a model for both NSR and the pSIE that includes island age as an additional fixed effect for the subset of oceanic islands where an age estimate was available (221 islands). We followed the General Dynamic Model5 of island biogeography and included island age as Age + Age2.
To validate the fitted models, we checked for collinearity in predictors via variance inflation factor (VIF) scores, with a threshold of less than five, and checked the residuals with the DHARMA package93, which simulates the standardized residuals from the fitted model and also checks for overdispersion and zero inflation. DHARMa reports statistical evidence of non-uniformity in the QQ plot. The plots themselves indicated that the effect size of these deviations from the expected distribution is small, and the significance of the deviation may be caused by a large number of data points (see Supplementary Fig. 2).
Island radiations within Asteraceae
We conducted a literature review of island radiations within Asteraceae to (1) synthesize our understanding of island radiations in Asteraceae, how many radiations there are and where they occur, (2) identify common characteristics shared between radiations, and (3) highlight understudied clades and regions that are promising for future research. Radiations are generally defined as rapid increases in the diversity of a lineage94. In the context of island biogeography, a radiation is typically considered to be the differentiation of a significant number of species in a short period of time through in situ cladogenetic speciation (via lineage splitting) occurring within an island region, from a single common ancestor that colonized an island or (meta-)archipelago. Radiations are often categorized as adaptive or non-adaptive based on a series of criteria95. In this study, we were interested in both types of radiations, as together they represent the diversity of cladogenetic mechanisms in the family, and we, therefore, include both types and record radiation type strictly as assessed by the primary publication.
In our literature search, we considered an island radiation to include three or more endemic species that are geographically restricted to an island or archipelago(s), and which result from a single colonization event and thus share a common ancestor. While our primary goal was to synthesize knowledge on the diversity of phylogenetically confirmed insular radiations within the family, we also wanted to highlight potential understudied radiations that are promising groups for future research. To this end, our review included both confirmed and putative radiations. Confirmed radiations were backed up by a well-sampled published phylogeny of the island taxa and mainland relatives, which has confirmed the island endemics to form a clade resulting from a single colonization event, that is, they are not the product of multiple colonizations from the mainland96,97. Putative radiations were defined as having at least three endemic species from a genus occurring on an island or archipelago but have not yet been fully sampled in a phylogeny; this designation is based on taxonomy alone and indicates the need for future phylogenetic research. By focusing on genera in our definition of radiation, we run the risk of missing insular radiations that are composed of multiple genera (e.g., as is the case for the confirmed Hawaiian silversword alliance radiation) when they originated by single colonization (i.e., single ancestry). For both confirmed and putative cases, radiating clades distributed across multiple archipelagos were considered as one insular radiation. For example, the Polynesian Bidens, which are distributed across the Hawaiian, Marquesas, Society, and Austral Islands all result from a single colonization of the Pacific islands and were considered a single insular radiation98. While delimiting radiations to their widest island range could conceal the subsequent inter-regional radiations (e.g., the 20 monophyletic Bidens on Hawai‘i), we included the archipelago and island distribution in our review to retain this information. For summary and visibility purposes, we grouped radiations into wider regions composed of groups of islands and archipelagos, which are defined in Supplementary Data 4.
To identify insular radiations, we took a twofold approach. First, we carried out a literature search in Google Scholar using the keywords (Asteraceae OR Compositae) AND Island AND Radiation. Second, we searched through the Island Asteraceae Checklist and filtered out genera with at least three endemic species on an island or archipelago. The checklist has a major advantage in helping to identify unknown or understudied potential radiations that would otherwise not be captured in the traditional literature search. With the list of candidate radiations, we manually assessed each potential case. If the radiation met our above criteria for “confirmed” radiation, we collected data on the geographic distribution, island type, taxonomy, number of species, type of radiation (i.e., adaptive or non-adaptive; as assessed by the original publication), traits often hypothesized to be associated with island radiations (breeding system, dispersal syndrome, ploidy level, hybridization), crown age, phylogenetic work done on the clade, and references. Characteristics and traits were collected at the radiation level. If species in a radiation had different traits, the radiation was marked as multi-state; for example, the Lipochaeta-Melanthera radiation on Hawai‘i is made up of both diploids and polyploids, and so we listed the ploidy level of this radiation as mixed. When we could not confirm the radiation through a well-sampled phylogeny, but taxonomic evidence indicated the group of endemic species might be a radiation, we marked the group as “putative radiation” and collected data on the geographic distribution, island type, taxonomy, potential number of species, and references.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All the data that support the findings of this study are provided alongside the paper in the Supplementary Data and include: (Supplementary Data 1) Global checklist of Asteraceae native and endemic to islands; (Supplementary Data 2) References for the global island Asteraceae checklist; (Supplementary Data 3) Dataset of islands with the number of native and endemic Asteraceae species and associated abiotic variables used in the mixed effects models; (Supplementary Data 4) Review of confirmed and putative island radiations in Asteraceae. Source data are provided with this paper.
Code availability
R code to run all analyses is available on GitHub at https://github.com/Lizzie-Roeble/Macroecology_Island_Asteraceae and archived at https://doi.org/10.5281/zenodo.11119678.
References
Govaerts, R., Lughadha, E. N., Black, N., Turner, R. & Paton, A. The world checklist of vascular plants, a continuously updated resource for exploring global plant diversity. Sci. Data 8, 215 (2021).
MacArthur, R. H. & Wilson, E. O. The Theory of Island Biogeography (Princeton Univ. Press, 1967).
Carlquist, S. Island Biology (Columbia Univ. Press, 1974).
Losos, J. B. & Ricklefs, R. E. (eds.) The Theory of Island Biogeography Revisited (Princeton Univ. Press, 2010).
Whittaker, R. J., Triantis, K. A. & Ladle, R. J. A general dynamic theory of oceanic island biogeography. J. Biogeogr. 35, 977–994 (2008).
Warren, B. H. et al. Islands as model systems in ecology and evolution: prospects fifty years after MacArthur-Wilson. Ecol. Lett. 18, 200–217 (2015).
Whittaker, R. J., Fernández-Palacios, J. M., Matthews, T. J., Borregaard, M. K. & Triantis, K. A. Island biogeography: taking the long view of nature’s laboratories. Science 357, eaam8326 (2017).
Gillespie, R. G. et al. Comparing adaptive radiations across space, time, and taxa. J. Heredity 111, 1–20 (2020).
Kier, G. et al. A global assessment of endemism and species richness across island and mainland regions. Proc. Natl. Acad. Sci. USA 106, 9322–9327 (2009).
Cai, L. et al. Climatic stability and geological history shape global centers of neo- and paleoendemism in seed plants. Proc. Natl. Acad. Sci. USA 120, e2300981120 (2023).
Kim, S.-C. et al. Timing and tempo of early and successive adaptive radiations in Macaronesia. PLoS ONE 3, e2139 (2008).
Givnish, T. J. et al. Origin, adaptive radiation and diversification of the Hawaiian lobeliads (Asterales: Campanulaceae). Proc. R. Soc. B Biol. Sci. 276, 407–416 (2008).
Fernández-Palacios, J. M. et al. A reconstruction of Palaeo-Macaronesia, with particular reference to the long-term biogeography of the Atlantic island laurel forests. J. Biogeogr. 38, 226–246 (2011).
Veron, S., Haevermans, T., Govaerts, R., Mouchet, M. & Pellens, R. Distribution and relative age of endemism across islands worldwide. Sci. Rep. 9, 11693 (2019).
Carlquist, S. Island Life: A Natural History of the Islands of the World (Natural History Press, 1965).
Burns, K. C. Evolution in Isolation: The Search for an Island Syndrome in Plants (Cambridge Univ. Press, 2019).
Taylor, A., Keppel, G., Weigelt, P., Zotz, G. & Kreft, H. Functional traits are key to understanding orchid diversity on islands. Ecography 44, 703–714 (2021).
Veron, S. et al. High evolutionary and functional distinctiveness of endemic monocots in world islands. Biodivers. Conserv. 30, 3697–3715 (2021).
Kreft, H., Jetz, W., Mutke, J., Kier, G. & Barthlott, W. Global diversity of island floras from a macroecological perspective. Ecol. Lett. 11, 116–127 (2008).
Weigelt, P. et al. Global patterns and drivers of phylogenetic structure in island floras. Sci. Rep. 5, 12213 (2015).
Carvajal-Endara, S., Hendry, A. P., Emery, N. C. & Davies, T. J. Habitat filtering not dispersal limitation shapes oceanic island floras: species assembly of the Galápagos archipelago. Ecol. Lett. 20, 495–504 (2017).
König, C. et al. Source pools and disharmony of the world’s island floras. Ecography 44, 44–55 (2021).
Grossenbacher, D. L. et al. Self-compatibility is over-represented on islands. N. Phytologist 215, 469–478 (2017).
Nürk, N. M., Atchison, G. W. & Hughes, C. E. Island woodiness underpins accelerated disparification in plant radiations. N. Phytologist 224, 518–531 (2019).
Zizka, A. et al. The evolution of insular woodiness. Proc. Natl. Acad. Sci. USA 119, e2208629119 (2022).
Barajas-Barbosa, M. P. et al. Assembly of functional diversity in an oceanic island flora. Nature 619, 545–550 (2023).
Fernández-Palacios, J. M. et al. Evolutionary winners are ecological losers among oceanic island plants. J. Biogeogr. 48, 2168–2198 (2021).
The Plant List (Version 1.1). http://www.theplantlist.org/ (2013).
Gostel, M. & Bonifacino, M. Global compositae database: a powerful new taxonomic resource. TAXON 69, 1137–1138 (2020).
Funk, V. A., Susanna, A., Stuessy, T. F. & Robinson, H. Classification of Compositae. in Systematics, Evolution, and Biogeography of Compositae (eds. Funk, V. A., Susanna, A., Stuessy, T. F. & Bayer, R. J.) Ch. 11, 171–189 (International Association for Plant Taxonomy, 2009).
Bernardello, G., Anderson, G. J., Stuessy, T. F. & Crawford, D. J. The angiosperm flora of the Archipelago Juan Fernandez (Chile): origin and dispersal. Botany 84, 1266–1281 (2006).
Antonelli, A. et al. Madagascar’s extraordinary biodiversity: evolution, distribution, and use. Science 378 (2022).
Lenzner, B., Weigelt, P., Kreft, H., Beierkuhnlein, C. & Steinbauer, M. J. The general dynamic model of island biogeography revisited at the level of major flowering plant families. J. Biogeogr. 44, 1029–1040 (2017).
Crawford, D. J. et al. Genetic diversity in Asteraceae endemic to oceanic islands: Baker's Law and polyploidy. in Systematics, Evolution, and Biogeography of Compositae (eds. Funk, V. A., Susanna, A., Stuessy, T. F. & Bayer, R. J.) Ch. 9, 139–147 (International Association for Plant Taxonomy, 2009).
Jeffrey, C. Evolution of Compositae flowers. in Systematics, Evolution, and Biogeography of Compositae (eds. Funk, V. A., Susanna, A., Stuessy, T. F. & Bayer, R. J.) Ch. 8, 131–138 (International Association for Plant Taxonomy, 2009).
Carlquist, S. The biota of long-distance dispersal. II. Loss of dispersibility in Pacific Compositae. Evolution 20, 30 (1966).
Heleno, R. & Vargas, P. How do islands become green? Glob. Ecol. Biogeogr. 24, 518–526 (2015).
Crawford, D. J., Kelly, J. K. & Anderson, G. J. Reproductive biology of Asteraceae on Oceanic Islands. Bot. Rev. 90, 67–108 (2024).
Schenk, J. J. The next generation of adaptive radiation studies in plants. Int. J. Plant Sci. 182, 245–262 (2021).
Cerca, J. et al. Evolutionary genomics of oceanic island radiations. Trends Ecol. Evol. 38, 631–642 (2023).
Fernández-Mazuecos, M. et al. The radiation of Darwin’s giant daisies in the Galápagos Islands. Curr. Biol. 30, 4989–4998.e7 (2020).
Kim, S.-C., Crawford, D. J., Francisco-Ortega, J. & Santos-Guerra, A. A common origin for woody Sonchus and five related genera in the Macaronesian islands: molecular evidence for extensive radiation. Proc. Natl. Acad. Sci. USA 93, 7743–7748 (1996).
Sang, T., Crawford, D. J., Kim, S. & Stuessy, T. F. Radiation of the endemic genus Dendroseris (Asteraceae) on the Juan Fernandez Islands: evidence from sequences of the its regions of nuclear ribosomal DNA. Am. J. Bot. 81, 1494–1501 (1994).
Cho, M.-S. et al. Plastid phylogenomics of Dendroseris (Cichorieae; Asteraceae): insights into structural organization and molecular evolution of an endemic lineage from the Juan Fernández Islands. Front. Plant Sci. 11, 594272 (2020).
Baldwin, B. G. & Sanderson, M. J. Age and rate of diversification of the Hawaiian silversword alliance (Compositae). Proc. Natl. Acad. Sci. USA 95, 9402–9406 (1998).
Carlquist, S., Baldwin, B. & Carr, G.Tarweeds & Silverswords: Evolution of the Madiinae (Asteraceae) (Missouri Botanical Garden Press, 2003).
Landis, M. J., Freyman, W. A. & Baldwin, B. G. Retracing the Hawaiian silversword radiation despite phylogenetic, biogeographic, and paleogeographic uncertainty. Evolution 72, 2343–2359 (2018).
Knope, M. L., Morden, C. W., Funk, V. A. & Fukami, T. Area and the rapid radiation of Hawaiian Bidens (Asteraceae). J. Biogeogr. 39, 1206–1216 (2012).
Knope, M. L., Bellinger, M. R., Datlof, E. M., Gallaher, T. J. & Johnson, M. A. Insights into the evolutionary history of the Hawaiian Bidens (Asteraceae) adaptive radiation revealed through phylogenomics. J. Heredity 111, 119–137 (2020).
Strijk, J. S. et al. In and out of Madagascar: dispersal to peripheral islands, insular speciation and diversification of Indian Ocean daisy trees (Psiadia, Asteraceae). PLoS ONE 7, e42932 (2012).
Vitales, D. et al. The explosive radiation of Cheirolophus (Asteraceae, Cardueae) in Macaronesia. BMC Evolut. Biol. 14, 118 (2014).
White, O. W., Reyes-Betancort, J. A., Chapman, M. A. & Carine, M. A. Geographical isolation, habitat shifts and hybridisation in the diversification of the Macaronesian endemic genus Argyranthemum (Asteraceae). N. Phytologist 228, 1953–1971 (2020).
Katinas, L., Crisci, J. V., Hoch, P., Tellería, M. C. & Apodaca, M. J. Trans-oceanic dispersal and evolution of early composites (Asteraceae). Perspect. Plant Ecol. Evol. Syst. 15, 269–280 (2013).
Mandel, J. R. et al. A fully resolved backbone phylogeny reveals numerous dispersals and explosive diversifications throughout the history of Asteraceae. Proc. Natl. Acad. Sci. USA 116, 14083–14088 (2019).
Magallón, S. & Castillo, A. Angiosperm diversification through time. Am. J. Bot. 96, 349–365 (2009).
Panero, J. L. & Crozier, B. S. Macroevolutionary dynamics in the early diversification of Asteraceae. Mol. Phylogenet. Evol. 99, 116–132 (2016).
Valente, L. et al. A simple dynamic model explains the diversity of island birds worldwide. Nature 579, 1–5 (2020).
Valente, L. M., Phillimore, A. B. & Etienne, R. S. Equilibrium and non-equilibrium dynamics simultaneously operate in the Galápagos islands. Ecol. Lett. 18, 844–852 (2015).
Francisco-Ortega, J. et al. Caribbean Island Asteraceae: systematics, molecules, and conservation on a biodiversity hotspot. Bot. Rev. 74, 112–131 (2008).
Richards, A. J. Apomixis in flowering plants: an overview. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 358, 1085–1093 (2003).
Noyes, R. D. Apomixis in the Asteraceae: diamonds in the rough. Funct. Plant Sci. Biotechnol. 1, 207–222 (2007).
Haveman, R. Freakish patterns - species and species concepts in apomicts. Nord. J. Bot. 31, 257–269 (2013).
Matthews, T. J., Triantis, K. A. & Whittaker, R. J. (eds.) The Species-Area Relationship: Theory and Application (Cambridge Univ. Press, 2021).
Kisel, Y. & Barraclough, T. Speciation has a spatial scale that depends on levels of gene flow. Am. Nat. 175, 316–334 (2010).
Triantis, K. A., Guilhaumon, F. & Whittaker, R. J. The island species-area relationship: biology and statistics. J. Biogeogr. 39, 215–231 (2012).
Ohyama, L., Holt, R. D., Matthews, T. J. & Lucky, A. The species-area relationship in ant ecology. J. Biogeogr. 48, 1824–1841 (2021).
Losos, J. B. & Schluter, D. Analysis of an evolutionary species-area relationship. Nature 408, 847–850 (2000).
Heaney, L. R. Dynamic disequilibrium: a long-term, large-scale perspective on the equilibrium model of island biogeography. Glob. Ecol. Biogeogr. 9, 59–74 (2000).
Nakagawa, S., Johnson, P. C. D. & Schielzeth, H. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 14, 20170213 (2017).
Galbany-Casals, M. et al. Phylogenetic relationships in Helichrysum (Compositae: Gnaphalieae) and related genera: Incongruence between nuclear and plastid phylogenies, biogeographic and morphological patterns, and implications for generic delimitation. TAXON 63, 608–624 (2014).
Pelser, P. B. et al. Patterns and causes of incongruence between plastid and nuclear Senecioneae (Asteraceae) phylogenies. Am. J. Bot. 97, 856–873 (2010).
Keeley, S. C., Forsman, Z. H. & Chan, R. A phylogeny of the “evil tribe” (Vernonieae: Compositae) reveals old/new world long distance dispersal: support from separate and combined congruent datasets (trnL-F, ndhF, ITS). Mol. Phylogenet. Evol. 44, 89–103 (2007).
Siniscalchi, C. M., Loeuille, B., Funk, V. A., Mandel, J. R. & Pirani, J. R. Phylogenomics yields new insight into relationships within Vernonieae (Asteraceae). Front. Plant Sci. 10, 1224 (2019).
Illera, J. C., Rando, J. C., Melo, M., Valente, L. & Stervander, M. Avian island radiations shed light on the dynamics of adaptive and nonadaptive radiation. Cold Spring Harb. Perspect. Biol. a041451 (2024).
Marques, D. A., Meier, J. I. & Seehausen, O. A combinatorial view on speciation and adaptive radiation. Trends Ecol. Evol. 34, 531–544 (2019).
Meudt, H. M. et al. Polyploidy on islands: its emergence and importance for diversification. Front. Plant Sci. 12, 637214 (2021).
Pannell, J. R. et al. The scope of Baker’s law. N. Phytologist 208, 656–667 (2015).
Razanajatovo, M. et al. Autofertility and self-compatibility moderately benefit island colonization of plants. Glob. Ecol. Biogeogr. 28, 341–352 (2019).
Weigelt, P., König, C. & Kreft, H. GIFT - a global inventory of floras and traits for macroecology and biogeography. J. Biogeogr. 47, 16–43 (2020).
Denelle, P., Weigelt, P. & Kreft, H. GIFT—an R package to access the global inventory of floras and traits. Methods Ecol. Evol. 14, 2738–2748 (2023).
Susanna, A. et al. The classification of the Compositae: a tribute to Vicki Ann Funk (1947–2019). TAXON 69, 807–814 (2020).
Weigelt, P. & Kreft, H. Quantifying island isolation - insights from global patterns of insular plant species richness. Ecography 36, 417–429 (2013).
Dinerstein, E. et al. An ecoregion-based approach to protecting half the terrestrial realm. Bioscience 67, 534–545 (2017).
Karger, D. N. et al. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 4, 170122 (2017).
Ramírez-Barahona, S., Sauquet, H. & Magallón, S. The delayed and geographically heterogeneous diversification of flowering plant families. Nat. Ecol. Evol. 4, 1232–1238 (2020).
Zuur, A. F., Ieno, E. N. & Elphick, C. S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 1, 3–14 (2010).
Whitehead, D. R. & Jones, C. E. Small islands and the equilibrium theory of insular biogeography. Evolution 23, 171–179 (1969).
Schrader, J. et al. Species-area relationships on small islands differ among plant growth forms. Glob. Ecol. Biogeogr. 29, 814–829 (2020).
Stoklosa, J., Blakey, R. V. & Hui, F. K. C. An overview of modern applications of negative binomial modelling in ecology and biodiversity. Diversity 14, 320 (2022).
Brooks, M. E. et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R. J. 9, 378 (2017).
Rijsdijk, K. F. et al. Recent geospatial dynamics of Terceira (Azores, Portugal) and the theoretical implications for the biogeography of active volcanic islands. Front. Biogeogr. 12 (2020).
Price, J. P. & Clague, D. A. How old is the Hawaiian biota Geology and phylogeny suggest recent divergence. Proc. R. Soc. Lond. Ser. B Biol. Sci. 269, 2429–2435 (2002).
Hartig, F. DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. https://CRAN.R-project.org/package=DHARMa (2022).
Linder, H. P. Plant species radiations: where, when, why? Philos. Trans. R. Soc. B Biol. Sci. 363, 3097–3105 (2008).
Schluter, D. The Ecology of Adaptive Radiation (Oxford Univ. Press, 2000).
Igea, J., Bogarín, D., Papadopulos, A. S. T. & Savolainen, V. A comparative analysis of island floras challenges taxonomy-based biogeographical models of speciation. Evolution 69, 482–491 (2015).
Papadopulos, A. S. T. et al. Speciation with gene flow on Lord Howe Island. Proc. Natl. Acad. Sci. USA 108, 13188–13193 (2011).
Knope, M. L. et al. Dispersal and adaptive radiation of Bidens (Compositae) across the remote archipelagoes of Polynesia. J. Syst. Evol. 58, 805–822 (2020).
Acknowledgements
L.R. was funded by a Vidi grant from the Dutch Research Council awarded to L.V. (NWO 016.Vidi.189.006). H.K. acknowledges funding from the German Research Foundation (DFG grants 533271599 and 379417748). J.R.M. acknowledges funding from the U.S. National Science Foundation (IOS-2214472). Research by P.V. was funded by the Spanish project PIB2022-13790 6NB-100 (Ministerio de Ciencia, Innovación y Universidades).
Author information
Authors and Affiliations
Contributions
Conceptualization: L.R., K.J.v.B., P.W., H.K., M.L.K., J.R.M., P.V., R.S.E., and L.V.; Data curation: L.R., P.W., H.K., and L.V.; Formal analysis: L.R., K.J.v.B., R.S.E., and L.V.; Investigation: L.R. and L.V.; Methodology: L.R., K.J.v.B., R.S.E., and L.V.; Software: L.R., K.J.v.B., P.W., and H.K.; Visualization: L.R.; Writing—original draft: L.R. and L.V.; Writing—review & editing: L.R., K.J.v.B., P.W., H.K., M.L.K., J.R.M., P.V., R.S.E., and L.V.; Funding acquisition: L.V.; Supervision: R.S.E. and L.V.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Bruce Baldwin, José Bonifacino, Pamela Puppo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Roeble, L., van Benthem, K.J., Weigelt, P. et al. Island biogeography of the megadiverse plant family Asteraceae. Nat Commun 15, 7276 (2024). https://doi.org/10.1038/s41467-024-51556-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51556-7
- Springer Nature Limited