Abstract
Photosystem II (PSII) catalyzes the light-driven charge separation and water oxidation reactions of photosynthesis. Eukaryotic PSII core is usually associated with membrane-embedded light-harvesting antennae, which greatly increase the absorbance cross-section of the core. The peripheral antennae in different phototrophs vary considerably in protein composition and arrangement. Photosynthetic cryptophytes possess chlorophyll a/c binding proteins (CACs) that serve as their antennae. How these CACs assemble with the PSII core remains unclear. Here, we report the 2.57-Å resolution structure of cryptophyte PSII-CAC purified from cells at nitrogen-limited stationary growth phase. We show that each monomer of the PSII homodimer contains a core complex, six chlorophyll a/c binding proteins (CACs) and a previously unseen chlorophyll-binding protein (termed CAL-II). Six CACs are arranged as a double-layered arc-shaped non-parallel belt, and two such belts attach to the dimeric core from opposite sides. The CAL-II simultaneously interacts with a number of core subunits and five CACs. The distinct organization of CACs and the presence of CAL-II may play a critical role in stabilizing the dimeric PSII-CAC complex under stress conditions. Our study provides mechanistic insights into the assembly and function of the PSII-CAC complex as well as the possible adaptation of cryptophytes in response to environmental stresses.
Similar content being viewed by others
Introduction
Oxygenic photosynthesis converts light energy into chemical energy and releases oxygen, thus sustaining nearly all life on our planet. Photosystems I and II (PSI and PSII) comprise two pigment-protein complexes that are embedded into the thylakoid membrane of chloroplasts, where they play fundamental roles in the photosynthesis process1. PSII is the only known natural enzyme capable of carrying out the light-driven water-splitting reaction. Its core complex is highly conserved across different species2, where it comprises dozens of protein subunits as well as various cofactors, including chlorophylls (Chls), carotenoids (Cars), plastoquinones (PQs), and the Mn4CaO5 cluster (oxygen evolving center, OEC)3. During photosynthesis, light-induced charge separation occurs in a special chlorophyll pair called P680 located in the core4. The electrons are subsequently transferred through the internal electron transfer chain to the PQ molecule located at the QB site, which further diffuses into the thylakoid membrane and transfers electrons to downstream acceptors5,6. The oxidized P680+ is then reduced by electrons stripped from water molecules through the function of OEC.
In most phototrophs, the PSII core is usually associated with peripheral light-harvesting antennae, which are critical for increasing the light harvesting capability of the PSII core. In contrast to the highly conserved core complex, peripheral antennae vary considerably in different photosynthetic organisms, and are roughly divided into the membrane-extrinsic phycobiliproteins (PBPs) as well as the membrane-intrinsic light harvesting complexes (LHCs)2,7. LHCs are commonly comprised of Chl a/b binding proteins in green lineages of phototrophs, whereas in red lineages, LHCs constitute Chl a/c binding (CAC) proteins7,8. Moreover, these antenna proteins bind a large variety of carotenoids, presumably representing an adaptation mechanism to the specific ecological niches these phototrophs occupy.
Photosynthetic cryptophytes, belonging to the red lineage of phototrophs, presumably originated from red algae following secondary endosymbiosis events9. Recently, cryptophytes have received considerable attention for their peculiar antenna systems, which combine water-soluble PBPs located in the thylakoid lumen and membrane-intrinsic CACs10. These CACs harbor pigments including carotenoid alloxanthin and chlorophylls a and c, thus are also termed ACPs (alloxanthin chlorophyll a/c binding protein)11. In addition, a previous study suggested that the arrangement pattern of cryptophyte CACs differs from that of other PSII antennae12.
Algae including cryptophytes commonly confront various stress conditions when grow in natural environment13. Nitrogen (N)-limitation is one of the major abiotic stresses, which were demonstrated to readily cause the damage of the fragile PSII core and a decline in its photosynthetic activity14,15,16. Phototrophs have evolved multiple mechanisms of photoprotection that minimize the damage to the reaction center. For example, LHC proteins are able to dissipate excess solar energy as heat, which is usually termed non-photochemical quenching (NPQ)17. Moreover, phototrophs possess effective repair mechanisms that convert the damaged PSII into functional PSII18,19,20. N-limitation was suggested to affect the dynamic balance of the damage-repair cycle of PSII due to the decreased level of the de novo protein synthesis21,22. Earlier reports showed that in cryptophytes, PBP is rapidly degraded at N-limited stress, presumably to support the cellular nitrogen requirements23,24. Interestingly, one previous study on cryptophyte Guillardia theta indicated that the cross-sections of its PSII increased under N-limited conditions25, suggesting that more CACs bind to the core when few PBPs are present. Moreover, previous studies showed that NPQ in cryptophyte cells most likely occurs in CAC antennae rather than PBPs26,27. It could be deduced that under N-limited conditions, CACs bound to the PSII core may function in dissipating excess energy and protecting the core complex. A previous low-resolution 2D projection map of cryptophyte PSII-CAC revealed that the PSII-CAC complex adopts an architecture distinct from PSII-LHC complexes of other oxygenic phototrophs with known structures12, however, the detailed structure and assembly of cryptophyte PSII-CAC under N-limited stress remain unclear.
Here, we isolated the PSII-CAC complex from cryptophyte Rhodomonas salina cells at the N-limited stationary growth phase (RsPSII-CAC), and determined its structure at an overall resolution of 2.57-Å. Our structure reveals a distinct conformation, whereby CAC antennae form arc-shaped belts and are associated with the inactive dimeric PSII core.
Results
Photosynthetic performance and overall structure of RsPSII-CAC
One previous study demonstrated that as a result of the exponential growth of cryptophyte cells in the culture, nitrogen is nearly depleted in F/2 medium when cells enter the stationary growth phase28. In order to mimic a natural N-depleted stress condition, we cultured R. salina cells in F/2 medium for 15 days, and counted the cell numbers as well as measured nitrogen concentration in the culture medium every day. As shown in Supplementary Fig. 1a, we found that R. salina cells entered the stationary growth phase on day 6 and the nitrogen source in the culture medium is almost completely consumed at the same time. We also observed that the color of cells changed from red to yellow when transiting from the logarithmic to stationary growth phase (Supplementary Fig. 1b), which was attributed to the degradation of phycoerythrin 545 (PE545, one type of PBPs) in R. salina24. This phenomenon was regarded as an indicator for cryptophyte cells coping with nitrogen-depleted conditions23. From these results, we concluded that R. salina cells grown into the late stationary phase (cultured for 13 days) are under a N-depleted condition in our experiment.
In order to analyze the PSII activity of R. salina cells under normal and N-depleted conditions, we measured the maximum quantum yield (Fv/Fm) and oxygen evolution activity of cells in different growth phases. We found that the oxygen evolution rate and the Fv/Fm of cells in stationary phase decreased by ~30% compared to those in logarithmic phase (Supplementary Fig. 1c, d). These results indicated that R. salina cells at the N-depleted stationary phase exhibit decreased PSII performance, suggesting that a portion of PSII in R. salina cells under such conditions is in an inactive state. We purified the PSII-CAC complex from R. salina cells in the N-depleted stationary growth phase (Supplementary Fig. 2), and determined the RsPSII-CAC structure at an overall resolution of 2.57-Å using cryogenic electron microscopy (cryo-EM) (Fig. 1, Supplementary Fig. 3, 4 and Supplementary Table 1). On the basis of our high-quality cryo-EM density, together with the sequence information obtained from sequencing of the R. salina transcriptome, we were able to identify all subunits as well as most of the pigments, lipids and other cofactors present in the complex (Supplementary Table 2).
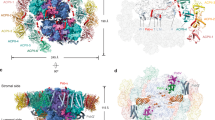
a, b Cryo-EM density of the RsPSII-CAC complex viewed from the stromal side (a) and from the side of the membrane plane (b). The PSII core, inner belt, outer belt and CAL-II are shown in different colors and indicated. c Structural model of RsPSII-CAC in the same view as in (a). Subunits in one PSII-CAC module are shown in different colors and labeled. For clarity, the small core subunits are labeled only in one letter with Psb being omitted. Subunits in another PSII-CAC module are shown in light yellow.
The RsPSII-CAC structure exhibits dimensions of approximately 250 × 180 × 90 Å3, displaying a hexagonal shape when viewed from the stromal side (Fig. 1a). Two identical modules of PSII-CAC form a homodimer (Fig. 1c), with each module comprising a monomeric PSII core, six peripheral CACs (designated as CAC1-6) and a distinct chlorophyll-binding protein that was not found in previously reported PSII supercomplexes. This protein links peripheral CACs to the PSII core, thus we termed it PSII Core-Antenna Linker protein (CAL-II). The PSII core consists of 16 membrane-intrinsic subunits (Fig. 1c), and binds 36 Chl a and 10 α-carotene molecules as well as other cofactors (Supplementary Table 2). While our core complex resembles the PSII core from other species (Supplementary Fig. 5), it lacks the Mn4CaO5 cluster and all lumenal extrinsic proteins as well as two small transmembrane subunits (PsbJ and PsbY). Six CACs constitute two antenna belts (hereafter termed the inner and outer belt) that attach to the dimeric core from one side in a non-parallel manner. The CAC belts slightly deviate from the thylakoid membrane plane and pivot towards lumen compared to the PSII core (Fig. 1b). CAL-II contains a long fragment spanning across the stromal surface of the inner CAC belt and the core complex, exhibiting an extended conformation. Interestingly, we found that six CACs assemble into one unit that binds to the dimeric PSII core. This type of antenna organization differs significantly from that of other previously reported PSII supercomplex structures, in which the peripheral antennae attached to one side of the PSII dimer can be divided into two parts, each of which bind to one of the two monomeric PSII cores29,30,31 (Supplementary Fig. 6).
Structures of CACs
Our high-quality cryo-EM density of the CAC belt region allows us to determine side chains of amino acids (Supplementary Fig. 7). Combining the results of our transcriptome sequencing, we were able to identify the six CACs, and found that they are six different gene products with high similarity in amino acid sequences (Supplementary Fig. 8). These six CACs adopt an overall architecture resembling each other, and share a common folding with other LHC proteins, containing three transmembrane (TM) helices (B, C and A) and two short amphipathic helices (E and D) (Fig. 2a). However, the conformation of the helix C as well as loop regions of six CACs differ visibly (Fig. 2a). We also found that helix C of both CAC3 and CAC6 are shorter than others, resulting in a longer loop between helix A and helix C (AC loop) (Fig. 2a). The different conformations adopted by these CACs may account for the formation of specific pigment-binding sites across various CACs, and are likely to be responsible for the differences observed for each CAC interacting with its neighboring subunits.
a Superposition of the apoproteins of six CACs. Three transmembrane helices and two lumenal helices are labeled. Different regions among six CACs are circled by dashed line. b Superposition of chlorophyll molecules in six CAC subunits. Conserved and nonconserved chlorophylls among six CACs are labeled in black and red, respectively. c Superposition of Chl c molecules in six CACs. The binding sites of these Chl c molecules are labeled. d Superposition of carotenoid molecules in six CACs. The type of carotenoids and their binding sites are labeled. The number in parentheses represents the number of the CAC protein (i.e., 1 indicates CAC1) in (b–d). The color codes for the six CAC subunits are the same as in Fig. 1c. The structure of CAC1 apoprotein is shown in transparent cartoon mode in (b–d).
We found that each CAC binds 11–14 chlorophylls (Fig. 2b, Supplementary Table 3); we assigned a total of 72 chlorophylls in six CACs. The Chl binding sites in CACs are similar to those in LHCII, except that CACs lack the binding site 608 (nomenclature is based on that used for spinach LHCII32), but contain an additional Chl binding site termed 615 (Fig. 2b, Supplementary Fig. 9). Of those 14 Chl binding sites, ten (Chls 602–604, 606, 609–613, and 615) are present in all CACs and reside in the relatively conserved transmembrane domain. The remaining four chlorophylls (Chls 601, 605, 607, and 614) bind to the peripheral loop regions of CACs (Fig. 2b), and are not all present in CACs except for CAC1.
Previous studies demonstrated that CACs bind both Chl a and Chl c, the latter extends the absorption of solar spectrum of CAC, thus facilitating light harvesting33. Since the two types of chlorophylls show similar chemical structure of the porphyrin ring, they are difficult to be distinguished solely based on their cryo-EM density feature. However, the hydrophobic phytol tail present in Chl a is replaced by a hydroxyl group in Chl c (Supplementary Fig. 4), thus Chl c may favor a more polar environment than Chl a. Based on our analysis of the densities and surrounding environment of all 72 chlorophylls, we tentatively assigned 12 Chls c, which are able to form hydrogen bonds with adjacent amino acids or pigments through their acrylate groups (Supplementary Fig. 10). The assigned Chl a/c ratio of CAC antennae in our structure is 5, which is similar to the previously reported Chl a/c ratio (4.4) of the purified CAC proteins associated with the PSII34. We found that these 12 Chls c in our RsPSII-CAC structure are unevenly distributed in six CACs, while CAC1 and CAC4 bind four and three Chls c, other four CACs only bind 1–2 Chls c (Fig. 2c, Supplementary Table 3).
In addition, our structure showed that each CAC binds five carotenoids located at the same sites (designated as 616–620) (Fig. 2d, Supplementary Table 3). These carotenoids may play important roles in light harvesting and/or photoprotection35. Of the five sites, 616 and 617 correspond to L1 and L2 sites in LHCII, and are conserved in all LHCs. These two carotenoids (Car 616 and Car 617) insert into the interior of CAC protein, suggesting that both carotenoids play critical roles in the process of CAC folding, similar to their counterparts found in LHCII36. In comparison, carotenoids 618-620 are located in the periphery of the CAC. In addition, Car 620 is positioned near parallel to Car 617 (Fig. 2d), with a distance between two carotenoids of ~5 Å.
Previous studies showed that cryptophyte CACs bind four types of carotenoids, namely alloxanthin (Alx), crocoxanthins (Cro), monadoxanthin (Mon), and α-carotene (α-Car). While the resolution of our cryo-EM reconstruction allows identification of Alx/Mon, Cro and α-Car (Supplementary Fig. 4), it does not allow for an unambiguous discrimination between Alx and Mon as their structure is nearly identical37. Both our HPLC analysis result (Supplementary Fig. 2d) and previously reported data37,38 showed that Alx is the major carotenoid in CACs. On the basis of these findings and our cryo-EM density feature, we tentatively assigned Alx in sites 616–619, and found that while site 620 is occupied by Cro in CAC1, CAC2, CAC4, and CAC5, it is likely occupied by α-Car in CAC3 and CAC6.
CAC-CAC organization and core-CAC interaction
Our structure showed that all six CACs present in one RsPSII-CAC module are arranged as two arc-shaped belts, each composed of three CACs. The two belts are arranged in a non-parallel manner, overlapping with each other on one end via CAC3 and CAC4. Moreover, we found that CAC located at the corresponding positions in these two belts (namely CAC-1 and CAC-4, CAC-2 and CAC-5, CAC-3 and CAC-6) exhibit high similarities in protein sequence, overall structure, and pigment arrangement (Fig. 3a, b, Supplementary Fig. 8b). As a result, the inner and outer CAC belts are well superposed, with an RMSD of 0.85 Å.
a, b Superposition of the CAC apoproteins in the inner and outer belt, viewed from the side of the membrane plane (a) and from the stromal side (b). The longer C-terminus of CAC5 is circled by black solid line in (a). The AC loops of CAC2/5/3/6 and the N-terminal fragments of CAC1/4/2/5 are colored in green and blue respectively in (b). c Typical interface of adjacent CAC subunits within the same belt (represented by CAC1-CAC2). Pigment and lipid molecules located in the interface are shown as sticks and labeled. d Interaction between CAC3 and CAC4, the two chlorophyll pairs at the interfaces are shown in stick-ball mode and labeled. e Cartoon representation of the RsPSII-CAC, viewed from stromal side. Subunits involved in core-CAC interactions are shown in different colors, and the interacting areas are boxed and shown in enlarged view in (f–i). f–i Protein–protein and protein–pigment interactions between CAC6 and PsbZ’ (f), CAC6 and CP43’ (g), CAC3 and PsbW’ (h), and CAC1 and PsbX (i). Side chains of amino acid residues, pigments, and lipids that are involved in the interactions are shown as sticks.
We also found that the interaction between neighboring CACs within the belt shows similar pattern, and primarily occurs at the stromal side as well as within the membrane plane (Fig. 3a–c). The N-terminus of one CAC (CACn+1) is closely associated with the AC loop of the adjacent CAC (CACn) at the stromal side (Fig. 3b). Moreover, pigment and lipid molecules located at the CAC-CAC interface, including Chl 606 of one CAC, and Chl 601, Chl 613, Alx 618 as well as the PG molecule coordinating Chl 611 from a neighboring CAC, strengthen the CAC-CAC interaction (Fig. 3c). While most CACs are only loosely associated with the adjacent ones at the lumenal side, we found that CAC5 possesses a longer C-terminal fragment that interacts with CAC4, thus stabilizing the outer belt at the lumenal side (Fig. 3a).
The two CAC belts are associated with each other through the Chl pairs 611–612 of CAC3 and 603–609 of CAC4 (Fig. 3d). Nevertheless, CACs from both belts interact with the core subunits of the PSII dimer (Fig. 3e–i). CAC1 and CAC3 in the inner belt simultaneously binds to PsbX and PsbW’ (where’ indicates the subunit from another module of the PSII dimer), respectively, and CAC6 in the outer belt associates with CP43’/PsbZ’. Notably, the longer AC loop of both CAC3 and CAC6 extends towards the core, thus maximizing their interactions with the PSII core from another module (Fig. 3g, h). Together, our structure strongly suggests that the double-layer arrangement of CACs plays a pivotal role in stabilizing the dimeric form of the RsPSII-CAC complex. The belt-shaped arrangement of peripheral antennae has never been found in PSII complex, indicating that the particular CAC organization is essential for the assembly and/or proper function of cryptophyte PSII complex.
Interestingly, we found that the two CAC belts in our RsPSII-CAC structure are aligning well with CAC belts of RsPSI-CAC structures and are also similar to LHCR belt of red algal PSI39 (Supplementary Fig. 11). Surprisingly, we found that CAC2 in our structure shares an identical sequence with one CAC protein (CAC-c) in RsPSI-CAC40. Together, these structural findings are in agreement with previous studies suggesting that the CAC proteins bound to both PSI and PSII in cryptophytes are evolutionarily originated from LHCR proteins, which serve as PSI antennae in red algae41.
The structure of CAL-II and its role in stabilizing RsPSII-CAC complex
The CAL-II was identified basing on our cryo-EM density and the sequencing analysis result (Fig. 4a, Supplementary Fig. 12), and its presence in the complex sample was verified by the mass spectrometry analysis (Supplementary Fig. 2b). The CAL-II comprises 284 amino acids, and binds two Chl a molecules. Homology searches in NCBI revealed that CAL-II homologs exist in various cryptophyte species, but can only be found in a few other eukaryotes with low coverage, which is in line with the fact that CAL-II has not been observed previously in other PSII structures.
a Overall structure and unsharpened cryo-EM map of CAL-II. The NTD and CTD are indicated. b Cartoon representation of one RsPSII-CAC module. CAL-II, helix C of CAC5, and CP47 are colored in blue, magenta, and salmon, respectively, whereas other subunits are colored in pink. Chls 301 and 302 of CAL-II and Chl a615 of CAC6 are shown in green color and labeled. c Interactions between CAL-II (shown in cartoon mode) and adjacent subunits (shown in surface mode). Subunits involved in the interaction with CAL-II are labeled and colored as in Fig. 1c. Other subunits are shown in white. d–j Hydrogen bond interactions between NTD of CAL-II and PsbH (d), CTD of CAL-II and PsbH (e), CTD of CAL-II and CP47 (f), CTD of CAL-II and D1 (g), CTD of CAL-II and CP43 (h), CTD of CAL-II and PsbT (i) and CTD of CAL-II and PsbM/PsbM’ (j). The PsbM from another PSII-CAC module (PsbM’) is colored in light yellow in (j). Hydrogen bonds are shown in blue dashed lines and the distances (Å) are labeled in the vicinity.
We modeled only the core part of CAL-II (P83-R173 and V205-G260) in our structure, and failed to identify any visible densities of the middle fragment that is composed of 31 amino acids (Fig. 4a). However, our SDS-PAGE analysis revealed that the CAL-II in the complex shows a molecular weight of ~31 kD (Supplementary Fig. 2b), indicating that it exists as an intact protein. Therefore, the missing density of the middle fragment of CAL-II is likely a result of its high mobility. The CAL-II protein in our structure can be divided into the N-terminal and C-terminal domain (NTD and CTD), which are connected by the middle mobile fragment (Fig. 4a). The NTD contains one TMH that is located between two CAC belts, exhibiting a nearly parallel orientation with the helix C of CAC5 (Fig. 4b, c). One of its chlorophyll (Chl 301) located adjacent to the TMH at the lumenal side, is positioned proximal to Chl 615 of CAC6 (Fig. 4b). Following the TMH, a long loop interconnects CAC3 and CAC4, and spans across the stromal surface of CAC3 (Fig. 4c). Two short helices and the other chlorophyll (Chl 302) insert into the interface between CP47 and CAC2/3 from the stromal side, where they form hydrogen bonds with the N-terminus of PsbH (Fig. 4b–d). The CTD of CAL-II is composed of loops and short helices, and its tail fragment forms a U-shaped turn. Multiple hydrogen bond interactions are formed between the CTD of CAL-II and core subunits including PsbH/CP47/D1/CP43/PsbT/PsbM (Fig. 4c–j). The CAL-II exhibits an extended overall architecture, with its stromal part stretching out from the position of the TMH to the PSII core for approximately 125 Å (Fig. 4c). These observations strongly suggest that the CAL-II subunit serves as a rope which ties the PSII core with the inner and outer belts, by forming numerous specific interactions with these three moieties, thus stabilizing the monomeric PSII-CAC module. In addition, CAL-II also contributes to the PSII dimer stabilization by directly interacting with PsbM’ from another monomer (Fig. 4c, j). Together, these results suggested that the essential role of CAL-II in cryptophytes is to stabilize and maintain the entire RsPSII-CAC complex.
Interestingly, while CAL-II homologs are absent in most other photosynthetic organisms, we found that the genome of the ciliate Tiarina fusa harbors a nearly identical CAL-II transcription product, based on a search of the NCBI Transcriptome Shotgun Assembly (TSA) database (Supplementary Fig. 12). This observation agrees with previous reports showing that ciliates ingest cryptophyte cells and retain the transcriptionally active nuclei and plastids of cryptophyte for a period of time42,43, providing additional evidence that cryptophytes and ciliates are photosymbiotically associated44.
Pigment arrangement in RsPSII-CAC
We next analyzed our structure for detailed pigment arrangement within the complex (Fig. 5), which allowed us to propose putative excitation energy transfer (EET) pathways and the potential quenching sites in RsPSII-CAC complex. We found that chlorophyll molecules in the complex are distributed into two layers located at the stromal and lumenal side. Twelve Chl c molecules are located at the peripheral region of the complex. Our structure showed that the Chls a and c are arranged in an alternate pattern, which may assist CACs to efficiently absorb sunlight across a wider light spectrum (Fig. 5a).
a Distribution of chlorophyll molecules and potential energy transfer pathways at the stromal side layer (left panel) and lumenal side layer (right panel) of RsPSII-CAC complex, viewed from the stromal side. Chls a and c are colored in green and blue. Chlorophylls belonging to different subunits and with Mg-to-Mg distances shorter than 20 Å are shown in bold stick and labeled. Energy transfer pathways within the CAC belts, between the outer and inner CAC belts and from the inner CACs to the PSII core are shown in red, blue, and purple dashed lines, respectively. b Distributions of carotenoid molecules in RsPSII-CAC complex, viewed from the stromal side. Alloxanthin (Alx), crocoxanthin (Cro), and α-carotene (α-Car) are colored in purple, orange, and light blue, respectively.
Excitation energy transfer largely depends on the Chl pairs with short distances and positioned at the interface between either CACs or CAC and the core. We observed that Chl a610 of CACn and Chl a601 of the CACn+1 are located at the CAC-CAC interface, thus may play a pivotal role for the EET within each CAC belt at the stromal side (Fig. 5a). At the lumenal side, Chl a606 of one CAC and Chl a613 of the adjacent CAC are closely associated with a distance of ~9 Å, which may enable efficient energy transfer between two neighboring CACs in each belt. The lumenal side EET within the CAC belt may also occur between the Chl c605 specific to CAC1 and CAC4 and Chl a613 of adjacent CAC. In addition, energy transfer within the outer CAC belt may be further facilitated by the Chl a301 of CAL-II, which is located proximal to Chl a607 of CAC5 and Chl 615 of CAC6 at the lumenal side (Fig. 5a). These multiple potential EET pathways may assist efficient energy equilibration within one CAC belt at both stromal and lumenal sides.
The inner belt is directly associated with the PSII core, which may enable efficient EET between inner CACs and the core. At the lumenal side, energy transfer could happen between interfacial Chl pairs, including CAC1-a615 and D2-a404, CAC1-a607 and CP47-a601, and CAC2-a615 and CP47-a601. At the stromal side, CAL-II-a302 is positioned at a short distance from both the CAC2-a603/a609 pair and CP47-a615/a616, thus may facilitate the energy transfer between the inner belt and core. To verify this assumption, we calculated the Förster resonance energy transfer (FRET) rates between CAC2-a603/a609 and CP47-a615/a616. The result showed that in the absence of CAL-II-a302, the EET from CAC2 to CP47 is inefficient, with a lifetime longer than 100 ps. In comparison, the CAL-II-302 mediates an efficient EET from CAC2 to CP47 through CAC2-a609/CAL-II-a302/CP47-a615 pathway, with a lifetime shorter than 5 ps (Table S4).
Due to the large spatial gap, EET between the outer belt and the core may be less efficient, and is likely mediated by the inner CAC belt. The energy exchange between the inner and outer CAC belts mainly occurs at the stromal side, namely between Chl a603/a609 pair of CAC4 and Chl a611/a612 pair of CAC3 (Fig. 5a).
Interestingly, we found that two chlorophylls (Chl c605 of CAC1 and CAC4) protrude further into the lumen than other chlorophyll molecules in the complex. This feature has not been observed in other structures of PSII complexes from both green and red lineage (Supplementary Fig. 13). Cryptophytes differ from other photosynthetic organisms in possessing lumen-localized antennae PBPs in addition to the membrane-intrinsic CACs. Thus, these two Chl c molecules may play an important role in mediating the energy transfer from lumen-localized PBPs to CAC antennae.
Carotenoids constitute a critical supplement to chlorophylls for the process of light harvesting, and also play important roles in dissipating excess excitation energy35. We analyzed the arrangement of 30 carotenoids bound to the six peripheral CACs in our RsPSII-CAC structure (Fig. 5b). These carotenoids are all arranged in close proximity to chlorophylls, where they form multiple Chl-Car pairs (Supplementary Fig. 14). This type of arrangement should allow carotenoids to deliver excitation energy to the neighboring chlorophylls effectively. Moreover, previous studies showed that Chl-Car interactions are involved in energy quenching mechanisms37,45, suggesting that these Chl-Car pairs in the RsPSII-CAC complex may also play a critical role in dissipating excess energy that is harmful to the photosynthetic machinery35. This suggestion is in agreement with previous reports showing that cryptophytes exhibit effective NPQ that is likely located in the CAC antennae, especially when the cryptophyte cells enter the stationary growth phase25,26,27.
Core features of RsPSII-CAC
Our structural analysis showed that our core complex lacks all lumenal extrinsic proteins, namely PsbO, PsbU, PsbV, PsbQ’, and Psb31, as well as two small transmembrane subunits PsbJ and PsbY (Fig. 6a). As their gene sequences were detected in our transcriptome sequence analysis, these proteins are likely present in the cells but became detached from the complex. In addition, our RsPSII-CAC structure showed that several core subunits adopt different conformation compared to those in the functional PSII. The differences primarily occur in the donor side (around the OEC) and regions proximal to PsbJ (Fig. 6, Supplementary Fig. 15a–g), whereas the acceptor side is almost unchanged (Supplementary Fig. 15h). Particularly, residues participating in Mn4CaO5 coordination show visible conformational changes compared to those in functional PSII (Fig. 6b). Moreover, we found that several membrane-intrinsic subunits (CP43, PsbE, PsbF, ycf12, and PsbZ) located adjacent to PsbJ in functional PSII moved away from the PsbJ position in our structure (Fig. 6c, Supplementary Fig. 15d–g), and the lumenal domain of CP43 also exhibits evident conformational change. In addition, the Cyt b559 heme, which is axially ligated by the N-terminal histidine residue from both PsbE and PsbF, exhibits longer His-Fe distances in our RsPSII-CAC structure (2.4 Å and 2.3 Å) when compared to that in functional PSII (2.2 Å and 2.0 Å) (Fig. 6d). Moreover, we failed to trace the N-terminus of PsbE in our RsPSII-CAC structure, indicating that this terminus is highly mobile. These features are usually observed in inactive PSII such as immature PSII or PSII assembly intermediates46,47,48, in which removing CP43 is a major step49. These structural observations suggested that our RsPSII-CAC complex harbors an inactive PSII core, presumably resulted from the stress caused by the N-depleted stationary phase. The mild purification procedure we used and the fact that the membrane-intrinsic subunit PsbJ is absent from the complex suggested that the formation of our RsPSII-CAC complex is unlikely to be an experimental artifact due to isolation, although we cannot completely rule out this possibility.
a Superposition of the PSII core from RsPSII-CAC structure with the red algal PSII core structure (PDB code: 7Y5E), viewed from the membrane plane. PSII core from RsPSII-CAC is shown in surface mode, subunits that are present in red algal PSII but lack in our PSII core (PsbO/PsbU/PsbV/PsbQ’/Psb31/PsbJ/PsbY) are shown in ribbon mode and labeled. b Superposition of the residues coordinating Mn4CaO5 cluster in RsPSII-CAC structure (shown in slate and sea green) and in native cyanobacterial PSII structure (PDB code: 3WU2, shown in gray). c Superposition of the PSII core from RsPSII-CAC structure (cyan) with red algal PSII (PDB code: 7Y5E, pink), viewed from the stromal side. Subunits that are different between the two structures are labeled. d Superposition of PsbE/PsbF and the heme molecule in RsPSII-CAC structure (shown in cyan) and in native PSII structure (PDB code: 3WU2, shown in gray), the distances of His-Fe bonds are labeled. e Density of the two ions and adjacent residues.
Interestingly, while our RsPSII-CAC complex does not contain the OEC, we observed two blebs of density located in negatively charged surficial pockets close to the Mn4CaO5 position (Fig. 6e). The densities may correspond to two metal ions, which we termed ion 1 and ion 2. Ion 1 is located between Asp170 and Glu189 of D1, similar to the metal ion previously observed in immature PSII structure47. This position was suggested to be the high-affinity site where the first Mn is bound in the process of photo-activation48. Ion 2 occupies the position corresponding to the sidechain of Mn4CaO5-coordinating residue His332 of D1 in functional PSII (Fig. 6b, e). These two ions may represent metal ions that are disassembled from the OEC cluster under stress and preserved in the close vicinity, which may allow a quick reassembly of intact Mn4CaO5 under suitable conditions. Meanwhile, other possibilities such that these two ions come from the solution buffer cannot be ruled out.
Discussion
Cryptophyte represents a distinct group of photosynthetic organisms that carry phycobiliproteins and CAC antenna proteins. The CACs are evolutionarily closely related to LHCs of red algae and distinguished from other LHCs by the presence of unusual carotenoids. In this study, we solved the high-resolution structure of cryptophyte RsPSII-CAC complex, presenting the assembly details of CACs and CAL-II with the PSII core, as well as the sophisticated pigment network within the complex. Our structure suggests that cryptophytes adopt a mechanism distinct from other phototrophs to assemble their peripheral antennae to the PSII core, and the CAL-II plays an essential role in this process. In addition, CAL-II also contributes to the efficient EET within the complex. The Chl 302 of CAL-II is located at the interface between CP47 and CAC2, greatly increasing the energy transfer from CACs to the core, as shown by our FRET rates calculation result. These structural features strongly suggest that CAL-II plays an essential role in energy exchange in addition to maintaining the RsPSII-CAC complex integrity. Furthermore, our structure revealed that two Chl c molecules, belonging to CAC1 and CAC4, protrude into the lumen and may thus function in mediating the energy transfer from lumenal PBPs to the PSII core/CACs.
It is noteworthy that although our purified RsPSII-CAC complex lacks OEC and is incapable of oxygen evolving, the R. salina cells in stationary phase still exhibit low oxygen evolution activity, suggesting the presence of other populations of active PSII complexes with OEC in stationary phase cells. Nitrogen-limitation in microalgae affects chloroplast proteins50,51, and causes a decline of PSII photosynthetic performance in cryptophytes52, consistent with our observation that cells in N-depleted stationary phase exhibit lower photosynthetic performance. These results suggested that N-depletion suppresses PSII repair (which may increase under N-replete conditions), resulting in the inactivation of a portion of PSII complex. Our RsPSII-CAC structure may represent the inactive population of PSII in N-depleted stationary phase cells.
Very recently, structures of PSII-ACPII (corresponding to our PSII-CAC complex) purified from logarithmic phase cells of another cryptophyte alga Chroomonas placoidea, were reported53,54. In addition to the CAC (termed ACPII in both CpPSII-ACPII structures) antennae and CAL-II (termed CCPII-S in ref. 53 and Psb-γ in ref. 54), the CpPSII-ACPII complex contains OEC, several membrane-extrinsic subunits and PsbJ, thus representing the active PSII form. Structural comparison indicated that CpPSII-ACPII complex (active PSII) and our RsPSII-CAC complex (inactive PSII) are similar to each other, however, the CpPSII-ACPII complex53 (PDB code: 8WB4) possesses three additional subunits/fragments (Unk1-3). These structural observations revealed the differences of PSII complexes in cryptophyte cells between logarithmic and stationary growth phase.
Despite a lack of OEC, our RsPSII-CAC structure showed that the peripheral CAC antennae are still attached to the core, presumably increasing the oxidative stress and thus resulting in further damage to PSII. However, our purification result showed that the RsPSII-CAC complex is one of the major populations of PSII in R. salina cells under N-depleted stress conditions, indicating that the RsPSII-CAC complex is considerably stable. We assumed that R. salina cells adopt effective photoprotective mechanisms that assist in alleviating the oxidative stress in N-depleted stationary phase, thus protecting the PSII from severe photodamage.
Previous studies demonstrated that peripheral antennae play dual roles in both light harvesting and photoprotection20,41,55,56. In plants and diatoms, the xanthophyll cycle or diadinoxanthin cycle carotenoids bound to their peripheral antenna proteins function in energy dissipation57,58. Cryptophytes do not contain xanthophyll or diadinoxanthin cycle carotenoids, instead, their CACs bind a large number of alloxanthin, which is the triple bond counterpart of zeaxanthin and was suggested to play a role in NPQ37. The high abundance of alloxanthin and the closely associated Chl-Car pairs observed in our structure may be critical for the protection function of cryptophytes, which could explain the previous observation that cryptophyte CACs have effective NPQ26. Moreover, secondary electron transfer within PSII was proposed to protect PSII against photodamage, with Cyt b559 being crucial in this process59. Our structure showed that the N-terminus of PsbE is mobile, which may change the electrostatic interactions of the heme propionates of Cyt b55959. Previous studies showed that changes of both His-Fe distances and the electrostatic environment of heme propionates may alter the redox potential of Cyt b559, allowing it to reduce the oxidized P680+. Furthermore, as PsbJ was shown to be essential for the transfer of PQ molecule60,61, the absence of PsbJ may prevent electrons from escaping out of the core, thus avoiding the loss of reduction equivalent, which may help the core to alleviate oxidative stress. These protective mechanisms may together assist cryptophytes to better adapt to perpetually changing natural environments.
Methods
Cell culture and photosynthetic activity measurements
Rhodomonas salina CCMP 1319 (purchased from Provasoli-Guillard National Center, https://ncma.bigelow.org) was cultured in artificial seawater62 based F/2 medium63 under continuous light (45–50 μmol photons m−2 s−1) at 21 °C with continuous bubbling of air. The growth curve of cells was plotted by counting cell numbers for 15 days (Fig. 1a). Result showed that cells are in L phase for the first 6 days and then enter S phase. According to study on culture of R. salina23, cells are under N-depleted conditions after entering S phase.
Cells cultured for five days (representing cells under normal conditions) and for thirteen days (representing cells under N-depleted conditions) were separately harvested and adjusted to same cell concentration (OD750nm 1.2) for further experiments. The oxygen-evolving activity was measured using a Chlorolab 2 oxygen measurement electrode system (Hansatech, England) at 25 °C. Cells were dark-adapted in the presence of 10 mM hydrogen carbonate as an electron acceptor for 2 min. Then O2 production was detected under illumination with a saturated white light of intensity of 3400 µmol m−2 s−1. Chlorophyll a fluorescence measurement was taken using an Imaging PAM (Heinz Walz, Germany). After dark adaptation for 15 min, cells were induced by a weak measuring light at a wavelength of 620 nm to determine the minimum fluorescence yield (Fo) just prior to the saturation pulse. The maximum fluorescence yield (Fm) was induced by the first saturation pulse (2300 µmol m−2 s−1). The maximum photochemical efficiency of PSII (Fv/Fm = (Fm−Fo)/Fm) was then calculated64.
RsPSII-CAC purification
The cells cultured at stationary phase (cultured for 13 days) were harvested by centrifugation at 6000 × g for 8 min. The pellet was suspended in buffer A (20 mM HEPES pH 7.5, 20 mM NaCl, 10 mM CaCl2, 10 mM MgCl2 and 1 M betaine) and broken using high pressure cell grinder under 750 bar. Unbroken cells were removed by centrifugation at 2500 × g for 5 min, and supernatant was further centrifuged at 70,000 × g for 40 min to obtain thylakoid membranes. To purify the PSII-CAC complex, the thylakoid membranes were resuspended in buffer A to a Chl concentration of 0.5 mg mL−1, and solubilized with 1.0% dodecyl-β-d-maltoside (β-DDM) at 4 °C for 10 min on ice. The solubilized mixture was centrifuged for 15 min at 13,500 × g, and the supernatant was loaded onto sucrose-density gradient (5–30%) in buffer A containing 0.02% α-DDM, followed by ultracentrifugation at 198,200 × g, 4 °C for 18 h using SW41 rotor (Beckman). The band at the lowest position of the gradient contains primarily the RsPSII-CAC complex and was collected (Supplementary Fig. 2a). The obtained sample were concentrated to 2 mg ml−1 (in chlorophyll) for cryo-EM specimen preparation.
Characterization of RsPSII-CAC
Room temperature absorption spectra of RsPSII-CAC was recorded using a spectrometer (Hitachi, Japan) (Supplementary Fig. 2c). Pigment composition (Supplementary Fig. 2d) was analyzed by high-performance liquid chromatography (HPLC) apparatus (Shimadzu, Japan) equipped with a RF20A prominence fluorescence detector. The RsPSII-CAC sample collected from the sucrose density gradient was concentrated to 1.5 mg ml−1 (in chlorophyll) and placed into 90% (v/v) cold acetone. The mixture was centrifuged at 13,000 × g for 10 min to extract pigments. The supernatant (10 μl) was auto-loaded on a Shim-pack GIST C18 reversed-phase column (Shimadzu, Japan), and the pigments were eluted at 18 °C at a flow rate of 1 ml/min with the following gradient: 0–20 min, linear gradient of buffer A (acetonitrile: water at the ratio of 85: 15) from 100% to 0%; 20–23 min, 100% buffer B (ethyl acetate); 23–24 min, linear gradient of buffer B from 100% to 0%; and 24–28 min, 100% buffer A. The eluent was detected at 440 nm with a detection range of 300–800 nm. Pigments were identified based on their absorption spectra and retention times of each peak fraction.
Protein subunits were separated by SDS–PAGE (Supplementary Fig. 2b). Coomassie bands in the SDS–PAGE were cut off separately for mass spectrometry (MS) analysis to identify the protein composition. MS analysis was performed using a matrix-assisted laser desorption ionization time-of-flight mass spectrometer (MALDI–TOF) (UltrafleXtreme, Brucker).
Amino acid sequencing
R. salina cells were harvested and frozen immediately in liquid nitrogen, then sent to Beijing Genomics Institute (BGI) for high-throughput sequencing analysis. Total RNA was extracted with a TRIzol Reagent according to the manufacturer’s instructions (Invitrogen, USA), and their purity and integrity were examined by gel electrophoresis, and Agilent RNA6000 Nano Reagents Kit (Agilent Technologies, USA), respectively. Complementary DNA (cDNA) library construction and bioinformatics analysis were performed by the technical staffs at BGI. The sequencing was repeated two times using cells under both normal and N-depleted conditions, and similar results were obtained.
Grid preparation and Cryo-EM data collection
Aliquots (3 μl) of the RsPSII-CAC sample were placed onto a holey carbon grid (GIG-C31213, 300M-Cu-R1.2/1.3) which was freshly glow-discharged for 40 s using an advanced plasma cleaning system (Gatan, Model 950) working at 25 W. The grid was blotted for 4 s with a blotting force of level 3 and then vitrified by flash plunging into liquid ethane using Vitrobot Mark IV (Thermo Fisher) set to 4 °C and 100% humidity. Cryo-grids were screened in a Talos F200C (Thermo Fisher) equipped with a CETA camera. The micrographs were imaged on a 300 kV Titan Krios microscope (Thermo Fisher) equipped with K2 direct electron detector (Gatan), using the semi-automatic program of SerialEM. The magnification of ×130,000 and the defocus range between −1.2 and −2.2 μm were used for data collection. Movie stacks were automatically acquired in the super-resolution mode with a pixel size of 0.52 Å. The total dose is about 60 electrons Å–2 in 32 frames.
Image processing
A total of 7875 raw movies were obtained and beam-induced motion was corrected using MotionCor2.165, producing two summed images with or without dose weighting. The parameters of the contrast transfer function on each summed image without dose weighting was determined using Gctf66. An initial set of 20,000 particles was manually picked and used as two-dimensional (2D) templates for automatic particle-picking in RELION3.167. As a result, 1,012,914 particles were auto-picked and subjected to one round (25 iterations) of 2D classification (Supplementary Fig. 3). Particles from good 2D classes were subjected to three-dimensional (3D) classification with a C1 symmetry using an initial model generated using RELION3.1 after lowpass filtering at 60 Å. The resultant 243,765 particles from the good 3D classes were subjected to 3D refinement with a C1 symmetry and post-processing, and then to CTF refinement and Bayesian polishing. The polished particles were employed for further 3D reconstruction of both the overall RsPSII-CAC and the peripheral inner and outer CAC belts.
For the overall reconstruction, no alignment 3D classification was applied to the 243,765 particles with a mask covering the CAC belts region with a C2 symmetry to remove particles harboring partial CAC belts, generating a good class containing 112,613 particles. These particles were used for refinement with a C2 symmetry and post-processing, and then to CTF refinement and final post-processing. The map of overall RsPSII-CAC was reconstructed at 2.57 Å resolution based on the gold-standard FSC with a cutoff value of 0.143. The local resolution of the final map was calculated using RELION 3.167.
To improve the resolution of the inner and outer CAC belts, the 243,765 particles were also performed no alignment 3D classification with C1 symmetry, leading to a good class containing 123,227 particles. The CAC belts region was finally reconstructed at 2.94 Å resolution through focus refinement with masking the CAC belt region, based on the gold-standard FSC with a cutoff value of 0.143. The final composite map used for model building was obtained by combining overall map and focused refinement map of CAC belts region using ‘vop maximum’ in Chimera68.
Model building and refinement
To construct a structural model of the PSII core moiety of RsPSII-CAC complex, the structure of the core of diatoms PSII-FCPII complex31 (PDB: 6JLU) was docked into the composite map using UCSF Chimera68, and the resulted structure was used as the initial model for further building and adjustment. The amino acid sequences of PSII core subunits were then mutated to their counterparts in R. salina obtained from unipro, except for PsaM. The sequence of RsPsaM was found in our transcriptome sequencing data.
To construct a structural model of each CAC subunit and the CAL-II, de novo model building was performed. We first manually built peptides with a length of five to eight amino acids in the transmembrane helix region, where amino acids show well resolved densities for side chains. Then we searched our transcriptome sequencing data using these peptide sequences to find matching proteins. The protein identities were further verified by examining the full sequence and the density for perfect match. Automatic real-space refinement using Phenix69 and manual correction using Coot70 were then performed interactively. The geometries of the final structures were assessed using MolProbity71. High-resolution images for publication were produced using Chimera X72.
FRET analysis
FRET rate constant was determined on the basis of FRET theory73. The formula for the FRET rate constant (kFRET) is kFRET = (CK2/(n4 R6), where C is a factor calculated from spectral overlap integral between the two Chls, K is the dipole orientation factor and n is the refractive index. The applied C value for Chl a to Chl a was 32.26 and value of n was 1.55, as estimated in ref. 74. The dipole orientation factor K2 was defined as K2 = [ûD · ûA − 3(ûD · RDA) (ûA · RDA)]2, where ûD and ûA are the unit vectors for the transition dipoles of the donor and the acceptor Chls derived from the vectors of the coordinates of NB and ND atoms, respectively, and RDA is the unit vector along the direction connecting the ‘central magnesium atoms’ from the donor Chl to the acceptor Chl. R was measured from the distance between central magnesium atoms of two Chls. FRET rates were computationally calculated using Kim’s algorithm on the Python platform (Python v.3.8).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The atomic coordinate of the RsPSII-CAC has been deposited in the Protein Data Bank under the accession code of 8XLP. The composite cryo-EM map and the overall cryo-EM map of the RsPSII-CAC supercomplex, as well as the locally refined cryo-EM map of the CAC moiety have been deposited in the Electron Microscopy Data Bank under accession codes of EMDB-38455, EMDB-38912, and EMDB-38913. Amino acid sequencing data have been deposited into the NCBI Sequence Read Archive (SRA) under the BioProject ID PRJNA1104392. All other relevant data generated in this study are provided in the Supplementary Information files and the Source data file. Source data are provided as a Source data file. The other structures used in this study are: 3WU2, 6JLU, 7Y5E, 8WB4, and 8XR6. Source data are provided with this paper.
References
Nelson, N. & Junge, W. Structure and energy transfer in photosystems of oxygenic photosynthesis. Annu. Rev. Biochem. 84, 659–683 (2015).
Croce, R. & Van Amerongen, H. Natural strategies for photosynthetic light harvesting. Nat. Chem. Biol. 10, 492–501 (2014).
Shen, J.-R. The structure of photosystem II and the mechanism of water oxidation in photosynthesis. Annu. Rev. Plant Biol. 66, 23–48 (2015).
Cardona, T., Sedoud, A., Cox, N. & Rutherford, A. W. Charge separation in photosystem II: a comparative and evolutionary overview. Biochim. Biophys. Acta (BBA)-Bioenerg. 1817, 26–43 (2012).
Ferreira, K. N., Iverson, T. M., Maghlaoui, K., Barber, J. & Iwata, S. Architecture of the photosynthetic oxygen-evolving center. Science 303, 1831–1838 (2004).
Holzwarth, A. R. et al. Kinetics and mechanism of electron transfer in intact photosystem II and in the isolated reaction center: pheophytin is the primary electron acceptor. Proc. Natl Acad. Sci. USA 103, 6895–6900 (2006).
Myśliwa-Kurdziel, B., Latowski, D. & Strzałka, K. Chlorophylls c—occurrence, synthesis, properties, photosynthetic and evolutionary significance. In Advances in Botanical Research, Vol. 90, 91–119 (Elsevier, 2019).
Buchel, C. Light harvesting complexes in chlorophyll c-containing algae. Biochim. Biophys. Acta Bioenerg. 1861, 148027 (2020).
Falkowski, P. G. et al. The evolution of modern eukaryotic phytoplankton. Science 305, 354–360 (2004).
Cunningham, B. R. et al. Light capture and pigment diversity in marine and freshwater cryptophytes. J. Phycol. 55, 552–564 (2019).
Doust, A. B., Wilk, K. E., Curmi, P. M. & Scholes, G. D. The photophysics of cryptophyte light-harvesting. J. Photochem. Photobio. A: Chem. 184, 1–17 (2006).
Kereïche, S. et al. Association of chlorophyll a/c2 complexes to photosystem I and photosystem II in the cryptophyte Rhodomonas CS24. Biochim. Biophys. Acta (BBA)-Bioenerg. 1777, 1122–1128 (2008).
Howarth, R. W. Nutrient limitation of net primary production in marine ecosystems. Annu Rev. Ecol. Syst. 19, 89–110 (1988).
Zhao, L.-S. et al. Nitrogen starvation impacts the photosynthetic performance of Porphyridium cruentum as revealed by chlorophyll a fluorescence. Sci. Rep. 7, 8542 (2017).
Bouchard, J., Longhi, M., Roy, S., Campbell, D. & Ferreyra, G. Interaction of nitrogen status and UVB sensitivity in a temperate phytoplankton assemblage. J. Exp. Mar. Biol. Ecol. 359, 67–76 (2008).
Rocha, G. S., Parrish, C. C. & Espíndola, E. L. Changes in photosynthetic parameters and lipid classes of N-limited Ankistrodesmus densus (Chlorophyceae) under cadmium exposure. J. Appl Phycol. 35, 99–107 (2023).
Muller, P., Li, X.-P. & Niyogi, K. K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 125, 1558–1566 (2001).
Takahashi, S. & Badger, M. R. Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci. 16, 53–60 (2011).
Johnson, V. M. & Pakrasi, H. B. Advances in the understanding of the lifecycle of photosystem II. Microorganisms 10, 836 (2022).
Horton, P. & Ruban, A. Molecular design of the photosystem II light-harvesting antenna: photosynthesis and photoprotection. J. Exp. Bot. 56, 365–373 (2005).
Allakhverdiev, S. I. & Murata, N. Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage–repair cycle of photosystem II in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta (BBA)-Bioenerg. 1657, 23–32 (2004).
Takahashi, S. & Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 13, 178–182 (2008).
Bartual, A., Lubián, L. M., Gálvez, J. & Niell, F. Effect of irradiance on growth, photosynthesis, pigment content and nutrient consumption in dense cultures of Rhodomonas salina (Wislouch) (Cryptophyceae). Cienc. Marinas 28, 381–392 (2002).
Yamamoto, S., Bossier, P. & Yoshimatsu, T. Biochemical characterization of Rhodomonas sp. Hf-1 strain (cryptophyte) under nitrogen starvation. Aquaculture 516, 734648 (2020).
Cheregi, O. et al. Presence of state transitions in the cryptophyte alga Guillardia theta. J. Exp. Bot. 66, 6461–6470 (2015).
Kaňa, R., Kotabová, E., Sobotka, R. & Prášil, O. Non-photochemical quenching in cryptophyte alga Rhodomonas salina is located in chlorophyll a/c antennae. PLoS ONE 7, e29700 (2012).
Kaňa, R., Kotabová, E., Šedivá, B. & Kuthanová Trsková, E. Photoprotective strategies in the motile cryptophyte alga Rhodomonas salina—role of non-photochemical quenching, ions, photoinhibition, and cell motility. Folia Microbiol. 64, 691–703 (2019).
Lafarga-De la Cruz, F. et al. Nutrient uptake, chlorophyll a and carbon fixation by Rhodomonas sp.(Cryptophyceae) cultured at different irradiance and nutrient concentrations. Aquacult. Eng. 35, 51–60 (2006).
Su, X. et al. Structure and assembly mechanism of plant C2S2M2-type PSII-LHCII supercomplex. Science 357, 815–820 (2017).
Sheng, X. et al. Structural insight into light harvesting for photosystem II in green algae. Nat. Plants 5, 1320–1330 (2019).
Pi, X. et al. The pigment-protein network of a diatom photosystem II–light-harvesting antenna supercomplex. Science 365, eaax4406 (2019).
Liu, Z. et al. Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428, 287–292 (2004).
Larkum, A., Ritchie, R. & Raven, J. Living off the Sun: chlorophylls, bacteriochlorophylls and rhodopsins. Photosynthetica 56, 11–43 (2018).
Bathke, L., Rhiel, E., Krumbein, W. & Marquardt, J. Biochemical and immunochemical investigations on the light‐harvesting system of the cryptophyte Rhodomonas sp.: evidence for a photosystem I specific antenna. Plant Biol. 1, 516–523 (1999).
Young, A. & Frank, H. Energy transfer reactions involving carotenoids: quenching of chlorophyll fluorescence. J. Photochem. Photobio. B: Biol. 36, 3–15 (1996).
Horn, R. & Paulsen, H. Folding in vitro of light-harvesting chlorophyll a/b protein is coupled with pigment binding. J. Mol. Biol. 318, 547–556 (2002).
Sebelik, V., West, R., Trskova, E. K., Kana, R. & Polivka, T. Energy transfer pathways in the CAC light-harvesting complex of Rhodomonas salina. Biochim Biophys. Acta Bioenerg. 1861, 148280 (2020).
De Oliveira-Júnior, R. G. et al. Carotenoids from Rhodomonas salina induce apoptosis and sensitize A2058 melanoma cells to chemotherapy. Rev. Bras. Farmacogn. 30, 155–168 (2020).
Pi, X. et al. Unique organization of photosystem I–light-harvesting supercomplex revealed by cryo-EM from a red alga. Proc. Natl Acad. Sci. 115, 4423–4428 (2018).
Zhang, S. et al. Growth phase-dependent reorganization of cryptophyte photosystem I antennae. Commun. Biol. 7, 560 (2024).
Kirilovsky, D. & Büchel, C. Evolution and function of light-harvesting antenna in oxygenic photosynthesis. In Advances in Botanical Research, Vol. 91, 247–293 (Elsevier, 2019).
Johnson, M. D., Oldach, D., Delwiche, C. F. & Stoecker, D. K. Retention of transcriptionally active cryptophyte nuclei by the ciliate Myrionecta rubra. Nature 445, 426–428 (2007).
Qiu, D., Huang, L. & Lin, S. Cryptophyte farming by symbiotic ciliate host detected in situ. Proc. Natl Acad. Sci. USA 113, 12208–12213 (2016).
Mordret, S. et al. The symbiotic life of Symbiodinium in the open ocean within a new species of calcifying ciliate (Tiarina sp.). ISME J. 10, 1424–1436 (2016).
Bode, S. et al. On the regulation of photosynthesis by excitonic interactions between carotenoids and chlorophylls. Proc. Natl Acad. Sci. USA 106, 12311–12316 (2009).
Xiao, Y. et al. Structural insights into cyanobacterial photosystem II intermediates associated with Psb28 and Tsl0063. Nat. Plants 7, 1132–1142 (2021).
Zabret, J. et al. Structural insights into photosystem II assembly. Nat. Plants 7, 524–538 (2021).
Gisriel, C. J. et al. Cryo-EM structure of monomeric photosystem II from Synechocystis sp. PCC 6803 lacking the water-oxidation complex. Joule 4, 2131–2148 (2020).
Heinz, S., Liauw, P., Nickelsen, J. & Nowaczyk, M. Analysis of photosystem II biogenesis in cyanobacteria. Biochim. Biophys. Acta 1857, 274–287 (2016).
Kumar Saha, S., Uma, L. & Subramanian, G. Nitrogen stress induced changes in the marine cyanobacterium Oscillatoria willei BDU 130511. FEMS Microbiol. Ecol. 45, 263–272 (2003).
Geider, R. J., Macintyre, H. L., Graziano, L. M. & McKAY, R. M. L. Responses of the photosynthetic apparatus of Dunaliella tertiolecta (Chlorophyceae) to nitrogen and phosphorus limitation. Eur. J. Phycol. 33, 315–332 (1998).
da Silva, A. F., Lourenço, S. O. & Chaloub, R. M. Effects of nitrogen starvation on the photosynthetic physiology of a tropical marine microalga Rhodomonas sp. (Cryptophyceae). Aquat. Bot. 91, 291–297 (2009).
Mao, Z. et al. Structure and distinct supramolecular organization of a PSII-ACPII dimer from a cryptophyte alga Chroomonas placoidea. Nat. Commun. 15, 4535 (2024).
Zhang, Y.-Z. et al. Structure of cryptophyte photosystem II–light-harvesting antennae supercomplex. Nat. Commun. 15, 4999 (2024).
Derks, A., Schaven, K. & Bruce, D. Diverse mechanisms for photoprotection in photosynthesis. Dynamic regulation of photosystem II excitation in response to rapid environmental change. Biochim. Biophys. Acta (BBA)-Bioenerg. 1847, 468–485 (2015).
Pinnola, A. & Bassi, R. Molecular mechanisms involved in plant photoprotection. Biochem. Soc. Trans. 46, 467–482 (2018).
Goss, R. & Jakob, T. Regulation and function of xanthophyll cycle-dependent photoprotection in algae. Photosynth. Res. 106, 103–122 (2010).
Lavaud, J., Rousseau, B. & Etienne, A.-L. In diatoms, a transthylakoid proton gradient alone is not sufficient to induce a non‐photochemical fluorescence quenching. FEBS Lett. 523, 163–166 (2002).
Chiu, Y.-F. & Chu, H.-A. New structural and mechanistic insights into functional roles of cytochrome b559 in photosystem II. Front Plant Sci. 13, 914922 (2022).
Choo, P. et al. The PsbJ protein is required for photosystem II activity in centers lacking the PsbO and PsbV lumenal subunits. Photosynth. Res. 1–9 (2021).
Müh, F., Glöckner, C., Hellmich, J. & Zouni, A. Light-induced quinone reduction in photosystem II. Biochim. Biophys. Acta (BBA)-Bioenerg. 1817, 44–65 (2012).
Kester, D. R., Duedall, I. W., Connors, D. N. & Pytkowicz, R. M. Preparation of artificial seawater 1. Limnol. Oceanogr. 12, 176–179 (1967).
Guillard, R. R. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals: Proceedings—1st Conference on Culture of Marine Invertebrate Animals Greenport 29–60 (Springer, 1975).
Consalvey, M., Perkins, R. G., Paterson, D. M. & Underwood, G. J. PAM fluorescence: a beginners guide for benthic diatomists. Diatom Res. 20, 1–22 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: Real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife 7, e42166 (2018).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr Sect. D: Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D: Biol. Crystallogr 66, 486–501 (2010).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. Sect. D: Biol. Crystallogr. 66, 12–21 (2010).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Mazor, Y., Borovikova, A., Caspy, I. & Nelson, N. Structure of the plant photosystem I supercomplex at 2.6 Å resolution. Nat. Plants 3, 1–9 (2017).
Gradinaru, C. C. et al. The flow of excitation energy in LHCII monomers: implications for the structural model of the major plant antenna. Biophys. J. 75, 3064–3077 (1998).
Acknowledgements
We are grateful to Z. Liu for insightful discussions. We thank C. Zhang from the Institute of Botany, CAS, for technical assistance in cell characterization; B. Zhu, X. Huang, and X. Li and other staff members at the Center for Biological Imaging (IBP, CAS) for their support in data collection; L. Niu for mass spectrometry; X. Zhao for assisting R. salina cell culturing; L. Shi for assisting pigment identification. We thank Torsten Juelich (University of Chinese Academy of Sciences) for linguistic assistance during the preparation of the article. The project is funded by the National Natural Science Foundation of China (31930064 to M.L.), the Strategic Priority Research Program of CAS (XDB37020101 to S.Z.), the CAS Project for Young Scientists in Basic Research (#YSBR-015 to X.S.) and Youth Innovation Promotion Association, Chinese Academy of Sciences (Y2022038 to X.S.).
Author information
Authors and Affiliations
Contributions
M.L. conceived and supervised the project; L.S. performed the sample preparation and characterization, cryo-EM data collection and processing, model building and refinement; S.Z. and X.S. assisted in sample preparation; L.S. and M.L. analyzed the data; L.S., S.Z., and M.L. wrote the manuscript; all authors discussed and commented on the results and the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Radek Kaña, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Si, L., Zhang, S., Su, X. et al. Structural basis for the distinct core-antenna assembly of cryptophyte photosystem II. Nat Commun 15, 6812 (2024). https://doi.org/10.1038/s41467-024-51206-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51206-y
- Springer Nature Limited