Abstract
Limited data from Asia are available on long-term effects of pneumococcal conjugate vaccine introduction on pneumococcal carriage. Here we assess the impact of 13-valent pneumococcal conjugate vaccine (PCV13) introduction on nasopharyngeal pneumococcal carriage prevalence, density and antimicrobial resistance. Cross-sectional carriage surveys were conducted pre-PCV13 (2015) and post-PCV13 introduction (2017 and 2022). Pneumococci were detected and quantified by real-time PCR from nasopharyngeal swabs. DNA microarray was used for molecular serotyping and to infer genetic lineage (Global Pneumococcal Sequence Cluster). The study included 1461 infants (5–8 weeks old) and 1489 toddlers (12–23 months old) enrolled from family health clinics. We show a reduction in PCV13 serotype carriage (with non-PCV13 serotype replacement) and a reduction in the proportion of samples containing resistance genes in toddlers six years post-PCV13 introduction. We observed an increase in pneumococcal nasopharyngeal density. Serotype 15 A, the most prevalent non-vaccine-serotype in 2022, was comprised predominantly of GPSC904;9. Reductions in PCV13 serotype carriage will likely result in pneumococcal disease reduction. It is important for ongoing surveillance to monitor serotype changes to potentially inform new vaccine development.
Similar content being viewed by others
Introduction
Infections due to Streptococcus pneumoniae (the pneumococcus) remain an important cause of childhood morbidity and mortality1 despite pneumococcal conjugate vaccine (PCV) introduction in 168 countries2. Pneumococcal carriage is a prerequisite for disease3 and community transmission. Reductions in carriage and transmission of vaccine serotypes to unvaccinated individuals4 results in the indirect effects of paediatric PCV vaccination5. Reductions in vaccine serotypes are associated with an increase in carriage and disease due to non-vaccine serotypes (serotype replacement)6. Although replacement can be extensive, there is usually a net benefit from vaccine introduction, as the replacing serotypes are generally less pathogenic7. However, in some settings this public health benefit of PCV has been eroded overtime6,8,9.
In low- and middle-income countries (LMICs) with limited invasive pneumococcal disease (IPD) surveillance, carriage is a useful outcome for evaluating the effects of PCV introduction10. Carriage studies are easier to conduct, as sample collection is less invasive than for IPD surveillance and sample size requirements are smaller. Serial cross-sectional community surveys in children are important to document direct and indirect effects on pneumococcal serotypes11. While several LMICs have conducted cross-sectional serial pneumococcal carriage surveys in healthy populations, only a handful of surveys are from Asia12,13,14,15,16 and none of these had data up to six years post-PCV introduction. Previously, we undertook community carriage surveys in Mongolia, comparing the pre-PCV13 (2015) period to one-year post-PCV13 introduction, finding a 50% reduction in PCV13 serotype carriage in infants and toddlers, with evidence of serotype replacement in toddlers17.
Although there is increasing evidence demonstrating the importance of pneumococcal nasopharyngeal density on disease and transmission, the effect of PCVs on pneumococcal density is largely unknown with heterogeneous findings from previous studies18. A systematic review explored antimicrobial resistance (AMR) of invasive and non-invasive paediatric pneumococcal isolates between 2000 and 2020 to understand the effect of PCV introduction on AMR19. Carriage isolates generally had a higher prevalence of penicillin and macrolide non-susceptibility than invasive isolates, where non-susceptibility was defined as an intermediate or resistant phenotype on antimicrobial susceptibility testing20. Over the 10 years following PCV introduction, reductions were estimated in non-susceptibility of multiple antibiotics. Changes in penicillin non-susceptibility were largely due to replacement of vaccine-targeted serotypes with non-vaccine serotypes19.
In this current analysis we aim to demonstrate the long-term impact of PCV13 introduction in Mongolia on pneumococcal carriage prevalence, density and AMR in the context of an established PCV13 program six years post-PCV introduction.
Results
Participant characteristics
There were 3000 children enrolled across the three surveillance years (2015, 2017, 2022). In 2015, 50 children did not meet the age inclusion criteria and were excluded from the analysis. All children met the study criteria in 2017 and 2022. Characteristics were similar between the first two surveys and have previously been described17. Here we compare the pre-PCV13 (2015) survey and the third survey (2022) which shows a reduction in the use of smoky fuels, fewer children living in traditional ger dwellings and less household members that smoked. The total number of people residing in each household increased (Table 1). In 2022 most (95%) toddlers aged 12-23 months had received three doses of PCV13.
Result from all three surveys
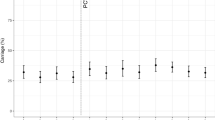
Figure 1 shows the carriage prevalence of all pneumococci, PCV13 serotypes and non-PCV13 serotypes across the three surveys. All pneumococcal and PCV13 carriage decreased over the three surveys. Compared with 2015, non-PCV13 carriage increased in 2017, and maintained that higher level in 2022. Comparing the final survey (2022) with the first post-PCV13 survey (2017) we observed a reduction in all pneumococcal carriage (25% in infants [aPR 0.75, 95%CI 0.58–0.96], 15% in toddlers [aPR 0.85, 95%CI 0.76–0.96]), a reduction in PCV13 serotype carriage (37% in infants [aPR 0.63, 95%CI 0.35–1.12], 35% in toddlers [aPR 0.65, 95%CI 0.48–0.90]) and a non-significant change in non-PCV13 serotypes (20% in infants [aPR 0.80, 95%CI 0.59–1.08] and 11% in toddlers [aPR 0.89, 95%CI 0.76–1.05]) (Supplementary Table S1).
A Carriage prevalence of all pneumococci, B 13-valent pneumococcal conjugate vaccine (PCV13) serotype pneumococci, and C non-PCV13 serotype pneumococci in two different age groups, in healthy children in Mongolia in 2015 (N = 950), 2017 (N = 999) and 2022 (N = 999). Error bars depict 95% confidence intervals for carriage prevalence (%). Source data are provided as a Source Data file.
When we compared the pre-PCV13 (2015) and the final post-PCV13 survey (2022), a reduction was observed in all pneumococcal carriage prevalence in both age groups, 33% in infants and 19% in toddlers (Table 2). In infants there was a 67% reduction in PCV13 serotype carriage (adjusted prevalence ratio [aPR] 0.33 [95% confidence interval [CI] 0.20–0.56]) compared with the pre-PCV13 period; but there was no significant increase in non-PCV13 serotype carriage prevalence (aPR 0.88 [95% CI 0.65–1.21]). In toddlers aged 12-23 months, there was a 71% reduction in PCV13 serotype carriage (aPR 0.29 [95% CI 0.22–0.38]), with evidence of serotype replacement (1.4 fold increase in non-PCV13 serotypes, aPR 1.36 [95% CI 1.12–1.65]).
Over all three years, serotyping results were obtained from 1132 of 1152 pneumococcal-positive samples (98%). A total of 20 samples could not be serotyped because they were either culture negative (n = 19) or had a low DNA yield from culture (n = 1). In all ages, most pneumococcal-positive samples contained a single serotype (963/1132, 85%). Only 8% of colonised infants (27/325) carried more than one serotype, while in contrast 18% (142/805) of colonised toddlers carried multiple serotypes (p < 0.001). In all infants there was no significant change in the prevalence of multiple serotype carriage over the three surveys: 3% (12/457), 2% (9/494) and 1% (6/496) (p = 0.27), but numbers were small. In toddlers the prevalence of multiple serotype carriage was similar in 2015 (64/488, 13%) and 2017 (60/497, 12%), with a substantial decrease observed in 2022 (18/494, 4%, χ2 p < 0.001). In participants who carried vaccine serotypes, these were not more likely to be present as a minor serotype in 2022 (compared with 2015 or 2017) for either infants or toddlers (Supplementary Table S2).
Prevalence of individual PCV13 and non-PCV13 serotypes fluctuated across the three surveys and varied by age group. For PCV13 serotypes, serotype 19F remained the most prevalent across all three years for infants (Fig. 2A), while in 12-23 month olds serotype 6A was replaced by 19F as the most prevalent serotype (Fig. 2B). Non-PCV13 serotypes showed a number of changes across the three years with several serotypes higher in 2017 than in 2022. Overall NT2 was the most prevalent non-PCV13 serotype in both age groups in 2015 and was replaced by 15A as most prevalent in infants (Fig. 2C) and toddlers (Fig. 2D) in 2022.
Results comparing first carriage survey (2015) and final carriage survey (2022)
The carriage prevalence of individual PCV13 serotypes and the most common non-PCV13 serotypes pre-PCV13 (2015) and post-PCV13 (2022) introduction are shown in Fig. 3 for infants and Fig. 4 for toddlers. In infants (Fig. 3) 45% (59/131) of pneumococci were PCV13 serotypes in the pre-PCV13 period, compared with 23% (20/87) in the post-PCV13 period (p = 0.003). In the pre-PCV13 period, the most common serotypes carried were 19F (n = 13), 23F (n = 13), non-encapsulated lineage NT2 (n = 12) and 6A (n = 10); while in the post-PCV13 period, serotypes 15A (n = 20), 19F (n = 11) and 10A (n = 9) predominated. For toddlers aged 12-23 months (Fig. 4), 70% (206/294) of pneumococci were PCV13 serotypes in the pre-PCV13 survey, compared with 26% (62/241) post-PCV13 introduction (p < 0.001). The most common serotypes identified in 2015 were 6A (n = 61), 19F (n = 47), non-encapsulated lineage NT2 (n = 30), 23F (n = 29) and 14 (n = 28) compared with 15A (n = 64), 19F (n = 35), 34 (n = 31), and 10A (n = 28) in 2022.
Solid bars indicate carriage that was detected as a single or major (dominant) serotype, open bars indicate carriage that was detected as a minor (second or third) serotype. NT2, NT3b and NT4b refer to different lineages of non-encapsulated pneumococci46. Other NVT includes all other identified non-PCV13 serotypes not listed individually. Source data are provided as a Source Data file.
Solid bars indicate carriage that was detected as a single or major (dominant) serotype, open bars indicate carriage that was detected as a minor (second or third) serotype. NT2, NT3b and NT4b refer to different lineages of non-encapsulated pneumococci46. Other NVT includes all other identified non-PCV13 serotypes not listed individually. Source data are provided as a Source Data file.
The density of pneumococcal carriage for all pneumococci, PCV13 serotypes, and non-PCV13 serotypes were higher in the final post-PCV13 survey (2022) compared with the pre-PCV13 period for both age groups (Supplementary Figs. S2A and S2B). In infants the median PCV13 carriage density was 4.71 (interquartile range [IQR] 3.98–5.61) log10 genome equivalents/ml (log10 GE/ml) in the pre-PCV13 period and 6.36 (IQR 5.40–7.04) log10 GE/ml in 2022. In toddlers the median PCV13 carriage density was 5.11 (IQR 4.23–5.75) log10 GE/ml in the pre-PCV13 period and 6.20 (IQR 5.42–6.85) log10 GE/ml in 2022 (Supplementary Table S3).
AMR genes were common, with 83% of samples containing at least one of the 10 AMR genes assessed. Samples with a PCV13 serotype were more likely to have at least one AMR gene detected (94.3%, 247/262) and multiple AMR genes (59.5%, 156/262), compared with samples with non-PCV13 serotypes (66.8%, 189/283 and 17.7%, 50/283, respectively) (Supplementary Table S3). Four AMR genes were significantly more common in PCV13 serotypes compared with non-PCV13 serotypes; tetM 92.0% versus 58.7%, cat 32.8% versus 14.1%, mefA 53.8% versus 19.1% and ermB 72.1% versus 41.3%, respectively (Supplementary Table S4). In infants 5–8 weeks, the proportion of samples containing cat decreased post-PCV13, while aphA3 and sat4 increased. There was a slight reduction in samples containing any AMR genes (81% to 79%), but not multiple resistance genes (Supplementary Table S5). In toddlers 12–23 months, the proportion of samples containing tetM, cat, mefA, ermB and ermC all decreased post-PCV13 introduction. There was a significant reduction in the proportion of samples containing any or multiple resistance genes in toddlers (Supplementary Table S5). With regards to the most common emerging non-PCV13 serotypes, for serotype 15A one (or more) AMR genes were detected in all isolates in the final survey in 2022 (40/40 [100%]) compared with a lower percentage in 2015 (3/4 [75%], p < 0.001). For serotypes 10A (7/24 [29%] versus 3/9 [33%], p = 0.91) and 34 (8/25 [32%] versus 6/14 [43%], p = 0.78) a lower percentage of isolates in the final survey had AMR genes compared with the previous two surveys.
Global Pneumococcal sequence clusters
Microarray data was available for 1132 pneumococcal positive samples and of these 1098 had a pneumococcus as the most abundant call and were analysed for lineage composition. We identified a total of 76 GPSCs from 1045 samples suitable for analysis. Serotype 19F remained the most prevalent vaccine serotype in 2022 and was comprised almost entirely of GPSC1 for both infants and toddlers across all three surveys (Fig. 5). Serotype 15A was the most prevalent non-vaccine serotype in 2022 and was comprised almost entirely of GPSC904;9 for both infants and toddlers across all three years. Serotype 10A and 34 were common non-vaccine-serotypes post-PCV13 introduction and were comprised almost entirely of GPSC634 and GPSC45, respectively, for both infants and toddlers across all three years (Fig. 5, Supplementary Figs. S3 and S4).
Global Pneumococcal Sequence clusters (GPSCs) were inferred for the calls with the highest relative abundance using DNA microarray and were analysed for lineage composition. Bars are coloured by age. Other refers to GPSCs that were found in fewer than five samples for the corresponding serotype. Source data are provided as a Source Data file (5–8 weeks N = 190, 12–23 months N = 533).
Discussion
This study aimed to describe the long-term effects of PCV13 on pneumococcal colonisation up to six years post-PCV13 introduction. We demonstrated that PCV13 introduction in the context of high vaccination coverage had a substantial impact on pneumococcal carriage in healthy children in Mongolia. PCV13 serotype carriage was reduced by around 70% in toddlers aged 12-23 months as well as for infants too young to be vaccinated, demonstrating substantial indirect vaccine effects.
Our study population had high PCV13 coverage (estimated at 96% in 202321) with extensive catch-up campaigns in children up to 24 months of age. Previously, we showed substantial reductions in PCV13 type pneumococcal carriage even at one year post-introduction17. In the current study reductions were maintained and increased in magnitude compared with the first post-PCV (2017) survey. Despite these reductions we found persistence of vaccine-types, with 12% of children carrying PCV13 type pneumococcal in 2022. These observations are consistent with our data in carriage in hospitalised children in Mongolia22. Serotype 19F was the most common vaccine type in the post-PCV period and was comprised exclusively of lineage GPSC1. This lineage is common in global datasets where it comprises serotypes 19A and 19F and less commonly, 3, 6C, 14 and 23F23. GPSC1 has been detected among serotype 19F isolates from South Africa, Peru, China and The Gambia23. The underlying mechanism behind the persistent carriage of certain serotypes such as 19F is still unclear.
Indirect protection is vital for young infants too young to be vaccinated as they are at high risk for pneumococcal disease. We observed the indirect herd effect of PCV13 on infants, with a 51% reduction in pneumococcal carriage already one year post introduction17 and a 67% reduction compared with the pre-PCV13 period in the current study. The high vaccine coverage and extensive catch-up campaign in the study districts may have contributed to the herd effect. Most LMIC studies reporting on indirect effects have demonstrated some evidence of herd effects, however, not all saw these changes in the infant age group11. Other studies with evidence of indirect effects in children aged <5 years showed reductions of 16–44% which was less than that observed in the current study11. Understanding indirect effects is important as taking protection of the unvaccinated population into consideration improves the cost-effectiveness of PCV use.
Although carriage surveys in healthy populations have been conducted in other countries from the WHO Western Pacific region12,13,14,15,16, these have focused on the immediate years following PCV introduction and there is little long-term evidence of vaccine impact and serotype replacement.
Reductions were observed in all these countries, except Papua New Guinea with very high carriage prevalence, density and multiple serotype carriage pre-PCV13 introduction, high serotype diversity and low vaccine coverage16. The degree of vaccine serotype carriage reductions varied in the other countries depending on the vaccine used, schedule, vaccine coverage, serotype distribution pre-vaccine and other population-specific factors12,13,14,15.
Many high-income countries saw a rapid reduction in vaccine serotype carriage post-PCV introduction24, while studies from Malawi25 and The Gambia26 reported residual vaccine serotype carriage 5-7 years post PCV introduction. Published community carriage studies from the WHO Western Pacific region, included post-PCV introduction periods ranging from 1-4 years12,13,14,15,16. In Nepal, four years post-PCV10 introduction, carriage prevalence of PCV10 serotypes was 5% and PCV13 serotypes 8% in children <2 years12. Monitoring the long-term changes in carriage serotypes helps to inform ongoing use of PCVs.
As expected, we observed serotype replacement with an increase in non-PCV13 carriage in vaccinated toddlers26,27. For both age groups non-vaccine serotypes 15A and 10A increased, and were common, post-introduction. The prevalence of serotype 34 also increased in toddlers. There was no increasing trend of replacement between the two post-PCV carriage surveys. Serotypes 10A, 34 and 15A have been identified as replacement serotypes in other studies26,27. Serotype replacement carriage relevance is dependent on the invasiveness of the increasing serotypes28. In hospitalised children with pneumonia in Mongolia, 15A, 15B/C and NT2 were the most common replacement serotypes22. Monitoring non-vaccine serotypes in different populations assists in understanding which populations are optimal to predict effects on disease. Of note is that serotype 15A and 34, which are emerging in Mongolia, are not included in any of the current higher valency vaccines (PCV15, PCV20 and PCV24)29. Lineage GPSC904;9 has been identified as associated with 15A-CC63 which is multidrug resistant30. The 15A-CC63 sub-lineage has been identified in several other countries including the USA, Israel and Hong Kong30.
The prevalence of infants (2%) and toddlers (10%) with multiple serotype carriage was low in our study. Multiple carriage is reported to promote genetic recombination. We observed a significant reduction in multiple serotype carriage from 13% (in 2015) to 4% (in 2022) in vaccinated toddlers. No substantial decrease was observed in the unvaccinated infant group, although baseline values were low. There is little evidence from LMICs on multiple serotype carriage as most methods only detect the dominant serotype. In contrast, we used microarray which is highly sensitive for detecting multiple serotype carriage31. The evidence of PCV impact on multiple serotype carriage is mixed although reductions have been observed in other studies32,33.
This study showed an increase in all pneumococcal, PCV13 and non-PCV13 density post-PCV introduction for both the early17 and long-term post-PCV surveys. Our systematic review which explored the impact of PCV on pneumococcal density found varying results between studies18. Previous studies in healthy children have shown both decreases13,34,35 and increases14,17 in carriage density with PCV introduction. Variations in density observed across our three cross-sectional surveys may be temporal and/or related to unmeasured factors. These factors may include viral co-infection, multiple serotype carriage, serotype replacement and prior antibiotic use. It is unclear which factors are driving the changes in pneumococcal density in Mongolia.
In our study, 83% of pneumococcal positive samples had at least one AMR gene, and samples with a PCV13 serotype were more likely to have at least one AMR gene detected. We observed a decrease in five AMR genes and in samples with multiple AMR genes in 2022 in vaccinated toddlers, which was not observed in the earlier post-PCV (2017) survey17. With regards to individual serotypes, it appears that overall antimicrobial resistance was not a factor in driving the emergence of non-vaccine serotypes. However, for serotype 15A the numbers in the pre-PCV13 period were small, and it is unclear what role antimicrobial resistance played in the increase in this serotype. PCV introduction has been shown to reduce resistance in circulating pneumococci19. Mongolia, like many other countries in Asia, has a history of inappropriate antibiotic prescription36. Increasing regulations have been introduced over the last decade towards limiting antibiotic use to prescription only. Changes in antibiotic use may have influenced our AMR findings. The most commonly used antibiotics in Mongolia include amoxicillin, cephalosporins and ciprofloxacin37,38.
Our final carriage survey was conducted in 2022 at a time when COVID-19 case numbers were low and only minimal pandemic related restrictions were in place39. Other studies in children have shown a reduction in IPD associated with pandemic restrictions, but no associated reductions in pneumococcal carriage40,41. The overall reductions in PCV13 type carriage observed in our 2022 carriage survey are likely predominantly due to PCV13 introduction and not non-pharmaceutical interventions (NPIs) introduced during the pandemic. It is unclear however whether NPIs or other factors may have played a role in the decrease of individual non-PCV13 serotypes in the final survey or whether these were cyclical changes in carriage prevalence unrelated to PCV13 introduction.
This study has several strengths. Firstly, our study was conducted in a country with a high number of risk factors for pneumococcal disease, located in Asia where there are limited data on PCV impact. Secondly, our study was conducted six years post-PCV introduction which is longer than other similar studies in the region. Thirdly, we sampled clinics randomly according to subdistrict to be representative of the different housing types in Ulaanbaatar, including to account for difference in health seeking behaviour. Our findings are therefore likely generalisable to other urban populations in Mongolia, as primary health care is free for children and immunisation coverage is high across the population. Lastly, we used sensitive molecular methods31,42 to measure the prevalence and density of pneumococcal carriage and were able to detect all pneumococcal serotypes present in a sample. Our additional GPSC analysis enables us to identify pneumococcal lineages that have emerged or persisted for non-vaccine type replacers and persistent vaccine-types allowing us to understand how PCV13 has induced changes within the pneumococcal population. Very few countries in Asia have included lineage analysis in PCV impact publications43,44. This study also has some limitations. Firstly, as mentioned above the final survey was conducted during the COVID pandemic, but this is unlikely to have substantially affected our results. Secondly, we did not collect information on all confounders that may potentially effect vaccine type carriage, for example air pollution levels and recent antibiotic use, and some of these factors may have resulted in us underestimating the impact of PCV on pneumococcal carriage. Although there was a reduction in the prevalence of some carriage risk factors in children included in the final survey, it is not possible to determine the relative contribution of these factors to changes in carriage rates. However, most of the reduction in carriage is likely driven by vaccine introduction based on observed reductions in vaccine-type serotypes and increases in non-vaccine type serotypes. Thirdly, AMR detection was limited to select resistance genes, and phenotypic testing was not conducted. Lastly, we inferred GPSCs and such inferences for samples with multiple serotype carriage may not always be accurate, although multiple carriage was relatively low in Mongolia.
This study provides evidence of substantial PCV impact on pneumococcal carriage in children up to six years after the introduction of PCV13 in Mongolia. It demonstrates persistent vaccine-types (especially 19F) and emerging non-vaccine type replacers that will be important to monitor in ongoing surveillance. In addition, it provides compelling data on AMR changes and will potentially inform new vaccine development through serotype changes.
Methods
Ethical approval
The study was approved by the Medical Ethics Review Committee at the Mongolian Ministry of Health and the Royal Children’s Hospital Human Research Ethics Committee (HREC 33203). Written informed consent was obtained from parents/caregivers prior to any study procedures being conducted.
Study site
Mongolia is a lower-middle income country with a population of around 3.4 million people in 2023. The Government of Mongolia introduced PCV13 into the routine national immunisation program using a 2 + 1 schedule (2, 4, 9 months) in a staged manner from 201645. PCV13 was introduced into the two survey districts (Songinokhairkhan and Sükhbaatar) in Ulaanbaatar in June 2016, with a catch-up campaign for children 3 to 23 months of age (two PCV13 doses, two months apart)45.
Study design and participants
Cross-sectional carriage surveys were conducted at family health centres in the two districts from May to July in 2015 (pre-PCV introduction), 2017 (one-year post-PCV introduction) and 2022 (six years post-PCV introduction). The methods and initial results for the first two surveys (2015 and 2017) have been previously published17. In brief, family health centre selection was stratified by predominant subdistrict housing type in each district and similar numbers of children were enrolled in each age group from each clinic. Only well children were invited to participate and children with respiratory symptoms were excluded. Children were ineligible if they had a fever, had not lived in one of the study districts for at three or more months (toddler group), or if they were infants and had received PCV1317. Staff completed questionnaires and collected nasopharyngeal swabs from all enrolled participants. PCV13 vaccination status was verified using the child’s vaccination card or clinic records.
Sample collection and laboratory procedures
World Health Organization recommended methods were followed for nasopharyngeal sample collection, handling and transport42. Swab processing and DNA extraction (MagNA Pure LC machine) for the earlier surveys (2015 and 2017) was previously described17. DNA extraction of swabs for the 2022 survey was performed using the QIAcube HT machine (QIAgen), lytA qPCR using the Stratagene Mx3005 machine, and molecular serotyping by microarray13,14,17. Microarray can determine the presence of multiple serotype carriage and the relative abundance of each pneumococcal serotype. The dominant serotype is considered the major serotype and the less abundant serotypes are designated as the minor serotypes31. PCV13 serotypes were defined as 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F. All other serotypes, including non-encapsulated pneumococci46, were designated non-PCV13 serotypes. Genetic lineage (using Global Pneumococcal Sequence Cluster, GPSC) was inferred from genomic profiling by microarray to examine lineage changes across the carriage surveys. AMR genes were also identified using microarray (Supplementary Methods page 1).
Statistical analysis
Sample size calculations were previously described; in brief we assumed that 281 participants in each age group with a baseline PCV13 serotype carriage prevalence of 16%, would detect a 50% reduction, with 90% power at a 5% significance level. Numbers were roughly doubled as true prevalence in 2015 was unknown17. Study data for the 2022 survey were collected and double data entered using the REDCap secure, web-based software platform hosted at the Murdoch Children’s Research Institute47. Databases were compared, cleaned and analysed using Stata version 17.0 and 18.0 (College Station, TX: StataCorp LLC). R (version 4.1.2) was used for the pneumococcal lineage analysis.
We used chi-squared tests to compare categorical data and Mann-Whitney test for continuous data.
Pneumococcal carriage prevalence was calculated for all, PCV13 and non-PCV13 serotypes for all years and for the most common individual serotypes. Unadjusted and adjusted prevalence ratios (aPR) were estimated using univariable and multivariable log-binomial regression by age group for all, PCV13 type and non-PCV13 type pneumococcal carriage prevalence for the pre-PCV13 (2015) and final post-PCV13 survey (2022) and for the two post-PCV13 surveys (2022 versus 2017). A common set of confounders was used to adjust the prevalence ratios comparing these various periods. The covariates were selected using a directed acyclic graph (Supplementary Fig. S1), informed by relevant literature, and included housing type (formal or informal), maternal education, household crowding (greater than three people per room), number of children under five years of age, household fuel type, and previous hospital admission. Reductions in PCV13 type carriage were calculated as (1 - aPR)*100%, while an increase in non-PCV13 type carriage was reported as a fold increase.
Pneumococcal carriage density data were log10 transformed and reported as log10 GE/ml. Median carriage densities were calculated for each age group (5-8 weeks and 12-23 months) for the pre-PCV13 (2015) and both post-PCV13 surveys (2017 and 2022). Median densities were compared using quantile regression to determine the impact of PCV13 introduction on pneumococcal density. A common set of confounders (Supplementary Fig. S1) was used to adjust the regression coefficient.
We determined detection rates of AMR genes for all, PCV13 type and non-PCV13 type pneumococci. We compared AMR detection rates between the pre-PCV13 and post-PCV13 periods for each age group. Samples with multiple resistance genes were considered as those with three or more AMR genes detected. Only samples that contained a single pneumococcal serotype with no other species identified were included in this analysis.
For genetic lineage analysis GPSCs were inferred for the serotypes detected with the highest relative abundance. Samples that had non-pneumococci detected as the highest relative abundance were excluded from further analysis. Data was stratified by year and age group. The number of samples belonging to each GPSC was plotted using R package ‘ggplot2’ (version 3.3.5).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All relevant data are within the paper and the supplementary information files. The source data underlying Figs. 1–5 and Supplementary Figs. S3&S4 are provided as a Source Data file with this paper. Additional data requests can be made to the corresponding author and should include details regarding the intended use of the data and appropriate approvals in line with local ethical requirements. Source data are provided with this paper.
Code availability
Data were analysed using Stata® version 17.0 and 18.0 (College Station, Texas, USA), with R (version 4.1.2) used for the pneumococcal lineage analysis.
References
Wahl, B. et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000-15. Lancet Glob Health 6, e744–e757 (2018).
International Vaccine Access Center (IVAC), Johns Hopkins Bloomberg School of Public Health. VIEW-hub 2023. Available from: www.view-hub.org. (Accessed 03 April 2023).
Simell, B. et al. The fundamental link between pneumococcal carriage and disease. Exp. Rev. Vaccines 11, 841–855 (2012).
Davis, S. M., Deloria-Knoll, M., Kassa, H. T. & O’Brien, K. L. Impact of pneumococcal conjugate vaccines on nasopharyngeal carriage and invasive disease among unvaccinated people: review of evidence on indirect effects. Vaccine 32, 133–145 (2013).
Klugman, K. P. Herd protection induced by pneumococcal conjugate vaccine. Lancet Glob Health 2, e365–e366 (2014).
Weinberger, D. M., Malley, R. & Lipsitch, M. Serotype replacement in disease after pneumococcal vaccination. Lancet 378, 1962–1973 (2011).
Løchen, A., Truscott, J. E. & Croucher, N. J. Analysing pneumococcal invasiveness using Bayesian models of pathogen progression rates. PLoS Comput. Biol. 18, e1009389 (2022).
Bertran, M. et al. Invasive pneumococcal disease 3 years after introduction of a reduced 1 + 1 infant 13-valent pneumococcal conjugate vaccine immunisation schedule in England: a prospective national observational surveillance study. Lancet Infect. Dis. 24, 546–556 (2024).
Mulholland, K. & Satzke, C. Serotype replacement after pneumococcal vaccination. Lancet 379, 1387 (2012).
Weinberger, D. M. et al. Using pneumococcal carriage data to monitor postvaccination changes in invasive disease. Am J Epidemiol 178, 1488–1495 (2013).
Chan, J. et al. Using pneumococcal carriage studies to monitor vaccine impact in low- and middle-income countries. Vaccine 37, 6299–6309 (2019).
Shrestha, S. et al. Effect of the of 10-valent pneumococcal conjugate vaccine in Nepal 4 years after introduction: an observational cohort study. Lancet Glob. Health 10, e1494–e1504 (2022).
Dunne, E. M. et al. Effect of ten-valent pneumococcal conjugate vaccine introduction on pneumococcal carriage in Fiji: results from four annual cross-sectional carriage surveys. Lancet Glob. Health 6, e1375–e1385 (2018).
Satzke, C. et al. Pneumococcal carriage in vaccine-eligible children and unvaccinated infants in Lao PDR two years following the introduction of the 13-valent pneumococcal conjugate vaccine. Vaccine 37, 296–305 (2019).
Turner, P. et al. Impact of 13-valent Pneumococcal conjugate vaccine on colonization and invasive disease in Cambodian children. Clin. Infect Dis. 70, 1580–1588 (2020).
Britton, K. J. et al. Lack of effectiveness of 13-valent pneumococcal conjugate vaccination against pneumococcal carriage density in Papua New Guinean infants. Vaccine 39, 5401–5409 (2021).
von Mollendorf, C. et al. Pneumococcal carriage in children in Ulaanbaatar, Mongolia before and one year after the introduction of the 13-valent pneumococcal conjugate vaccine. Vaccine 37, 4068–4075 (2019).
Jagne, I. et al. A systematic review of pneumococcal conjugate vaccine impact on pneumococcal nasopharyngeal colonisation density in children under 5 years of age. Vaccine 41, 3028–3037 (2023).
Andrejko, K., Ratnasiri, B., Hausdorff, W. P., Laxminarayan, R. & Lewnard, J. A. Antimicrobial resistance in paediatric Streptococcus pneumoniae isolates amid global implementation of pneumococcal conjugate vaccines: a systematic review and meta-regression analysis. Lancet Microbe 2, e450–e460 (2021).
CLSI. M100-ED29:2019 Performance Standards for Antimicrobial Susceptibility Testing 2019. Available from: https://clsi.org/standards/.
World Health Organization, UNICEF. Immunization 2024 Mongolia country profile. Available from: https://www.who.int/publications/m/item/immunization-2024-mongolia-country-profile (Accessed 20 July 2024).
von Mollendorf C., et al. Changes in Pneumococcal Carriage in Hospitalised Children 2-59 Months of Age in Mongolia Following Pneumococcal Conjugate Vaccine Introduction. Available from: https://doi.org/10.2139/ssrn.4488943
Gladstone, R. A. et al. International genomic definition of pneumococcal lineages, to contextualise disease, antibiotic resistance and vaccine impact. EBioMedicine 43, 338–346 (2019).
van Hoek, A. J. et al. Pneumococcal carriage in children and adults two years after introduction of the thirteen valent pneumococcal conjugate vaccine in England. Vaccine 32, 4349–4355 (2014).
Swarthout, T. D. et al. High residual carriage of vaccine-serotype Streptococcus pneumoniae after introduction of pneumococcal conjugate vaccine in Malawi. Nat Commun 11, 2222 (2020).
Usuf, E. et al. Persistence of nasopharyngeal pneumococcal vaccine serotypes and increase of nonvaccine serotypes among vaccinated infants and their mothers 5 Years after introduction of pneumococcal conjugate vaccine 13 in The Gambia. Clin Infect Dis 68, 1512–1521 (2019).
Hammitt, L. L. et al. Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and non-typeable Haemophilus influenzae in Kilifi, Kenya: findings from cross-sectional carriage studies. Lancet Glob Health 2, e397–e405 (2014).
Yildirim, I. et al. Serotype specific invasive capacity and persistent reduction in invasive pneumococcal disease. Vaccine 29, 283–288 (2010).
Rodgers, G. L., Whitney, C. G. & Klugman, K. P. Triumph of Pneumococcal Conjugate Vaccines: Overcoming a Common Foe. The Journal of Infectious Diseases 224, S352–S359 (2021).
Hawkins P. A., et al. A global genomic perspective on the multidrug-resistant Streptococcus pneumoniae 15A-CC63 sub-lineage following pneumococcal conjugate vaccine introduction. Microb Genom. 9.2023
Satzke, C., Dunne, E. M., Porter, B. D., Klugman, K. P. & Mulholland, E. K. PneuCarriage project group. The PneuCarriage project: a multi-centre comparative study to identify the best serotyping methods for examining pneumococcal carriage in vaccine evaluation studies. PLoS Med. 12, e1001903 (2015).
Valente, C. et al. Impact of the 13-valent pneumococcal conjugate vaccine on Streptococcus pneumoniae multiple serotype carriage. Vaccine 34, 4072–4078 (2016).
Valente, C. et al. Decrease in pneumococcal co-colonization following vaccination with the seven-valent pneumococcal conjugate vaccine. PLoS ONE 7, e30235 (2012).
Kandasamy, R. et al. Decline in pneumococcal vaccine serotype carriage, multiple-serotype carriage, and carriage density in Nepalese children after PCV10 introduction: A pre-post comparison study. Vaccine, (2024).
Roca, A. et al. Effect of age and vaccination with a pneumococcal conjugate vaccine on the density of pneumococcal nasopharyngeal carriage. Clin Infect. Dis. 55, 816–824 (2012).
Dorj, G., Hendrie, D., Parsons, R. & Sunderland, B. An evaluation of prescribing practices for community-acquired pneumonia (CAP) in Mongolia. BMC Health Serv. Res. 13, 379 (2013).
Dorj, G. et al. Antibiotic Utilization Trends in Two State Hospitals of Mongolia from 2013 to 2017. BioMed Res. Int. 2019, 9160296 (2019).
Dorj, G. et al. National surveillance of antibiotic consumption in Mongolia. Int. J. Infect. Dis. 101, 96 (2020).
Center for Health Development. Health Statistics Information Database: Ministry of Health, Mongolia; 2023. Available from: https://1313.mn/ (Accessed 06 Jan 2023).
Danino, D. et al. Decline in Pneumococcal disease in Young children during the coronavirus disease 2019 (COVID-19) pandemic in Israel associated With Suppression of Seasonal Respiratory Viruses, Despite Persistent Pneumococcal Carriage: A Prospective Cohort Study. Clin Infect Dis 75, e1154–e1164 (2022).
Rybak, A. et al. Association of Nonpharmaceutical Interventions During the COVID-19 Pandemic With Invasive Pneumococcal Disease, Pneumococcal Carriage, and Respiratory Viral Infections Among Children in France. JAMA Netw Open 5, e2218959 (2022).
Satzke, C. et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine 32, 165–179 (2013).
Belman S., et al. Genetic background of Cambodian pneumococcal carriage isolates following pneumococcal conjugate vaccine 13. Microbial Genomics, 8. 2022
Javaid N., et al. Strain features of pneumococcal isolates in the pre- and post-PCV10 era in Pakistan. Microbial Genomics. 2024;10.
La Vincente, S. F. et al. Evaluation of a phased pneumococcal conjugate vaccine introduction in Mongolia using enhanced pneumonia surveillance and community carriage surveys: a study protocol for a prospective observational study and lessons learned. BMC Public Health 19, 333 (2019).
Salter, S. J. et al. Variation at the capsule locus, cps, of mistyped and non-typable Streptococcus pneumoniae isolates. Microbiology 158, 1560–1569 (2012).
Harris, P. A. et al. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inf. 95, 103208 (2019).
Acknowledgements
The project was funded by the Gavi Alliance (contract number PP61690717A2). CS was supported by a NHMRC Career Development Fellowship (1087957) and a veski Inspiring Women Fellowship. Authors affiliated with MCRI were supported by the Victorian Government’s Operational Infrastructure Support Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We would like to acknowledge the Ministry of Health in Mongolia and the WHO country office for their support in this project. We would like to thank participating caregivers and children, study staff, family health clinic staff and laboratory staff in Mongolia. We would like to thank researchers and laboratory staff at MCRI. Thanks to Katherine Gould from the Bacterial Microarray Group, St. George’s University of London for technical advice regarding microarray.
Author information
Authors and Affiliations
Contributions
Conception/design of the work: E.K.M., C.V.M., C.S.; local study management, coordination and responsibility: T.M.; responsible for study oversight: C.V.M., T.M., B.T., D.N., S.D.; acquisition of clinical data: M.U., B.S., D.L., P.B., T.M.; oversight of pneumococcal carriage data acquisition: C.S., E.M.D., B.D.O., M.L.N.; acquisition of laboratory data: B.D.O., C.L.P., A.W.H., M.L.N.; interpretation of microarray data: J.H.; analysis of lineage data: P.S.; interpretation of lineage data: P.S., L.B., C.S.; devised the analysis plan: C.V.M., C.N., C.S.; data cleaning: T.M., P.B., C.V.M.; analysis of data: C.V.M.; drafting of the manuscript: C.V.M. All authors were involved in data interpretation and review of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
C.V.M., M.U., C.D.N., P.B., B.S., D.L., C.S., T.M., and E.K.M. are investigators on a Pfizer collaborative research project, exploring the impact of PCV13 introduction on adult pneumonia in Mongolia, outside this work. E.M.D. is currently employed by Pfizer. C.S., E.K.M., and C.D.N. are investigators on a Merck Investigator Studies Program grant funded by MSD outside this work. C.V.M. has participated in expert forums for Pfizer and MSD. C.S. has participated in forums and seminars for Pfizer and MSD. J.H. is co-founder and shareholder of BUGS Bioscience Ltd., a not-for-profit spin-out company of St George’s, University of London. The remaining authors declare no competing interest.
Peer review
Peer review information
Nature Communications thanks Katherine Gallagher, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
von Mollendorf, C., Mungun, T., Ulziibayar, M. et al. Effect of pneumococcal conjugate vaccine six years post-introduction on pneumococcal carriage in Ulaanbaatar, Mongolia. Nat Commun 15, 6577 (2024). https://doi.org/10.1038/s41467-024-50944-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-50944-3
- Springer Nature Limited