Abstract
The 2,2,2-trifluoroethoxy group increasingly features in drugs and potential tracers for biomedical imaging with positron emission tomography (PET). Herein, we describe a rapid and transition metal-free conversion of fluoroform with paraformaldehyde into highly reactive potassium 2,2,2-trifluoroethoxide (CF3CH2OK) and demonstrate robust applications of this synthon in one-pot, two-stage 2,2,2-trifluoroethoxylations of both aromatic and aliphatic precursors. Moreover, we show that these transformations translate easily to fluoroform that has been labeled with either carbon-11 (t1/2 = 20.4 min) or fluorine-18 (t1/2 = 109.8 min), so allowing the appendage of complex molecules with a no-carrier-added 11C- or 18F- 2,2,2-trifluoroethoxy group. This provides scope to create candidate PET tracers with radioactive and metabolically stable 2,2,2-trifluoroethoxy moieties. We also exemplify syntheses of isotopologues of potassium 2,2,2-trifluoroethoxide and show their utility for stable isotopic labeling which can be of further benefit for drug discovery and development.
Similar content being viewed by others
Introduction
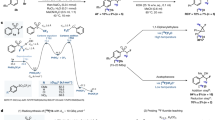
Trifluoromethylation finds extensive utility in medicinal chemistry and has led to many drug-like compounds with improved pharmacokinetic and physicochemical properties1. Over the past decade, substantial advances have been made in trifluoromethylation methods2,3,4. Fluoroform (HCF3) is a major industrial byproduct and now gains attention as an affordable and atom-efficient source of the trifluoromethyl group (CF3)5,6. Strategies for installing a trifluoromethyl group from fluoroform deploy nucleophilic or metal-mediated conversions (Fig. 1A). Nucleophilic trifluoromethylations rely on the deprotonation of fluoroform in the presence of a strong base to generate the reactive trifluoromethyl anion7, which can subsequently undergo reaction with a wide range of electrophiles8. Metal-mediated trifluoromethylation reactions9 convert fluoroform into a more stable copper(I) (CuCF310) or silver(I) (AgCF311) derivative, which can engage productively in diverse reactions.
Positron emission tomography (PET) is a molecular imaging modality that now plays a vital role in biomedical research, drug discovery, disease staging, and disease diagnosis12,13. PET tracers are produced at time of need and the majority are labeled with cyclotron-produced short-lived carbon-11 (t1/2 = 20.4 min) or fluorine-18 (t1/2 = 109.8 min)14,15,16.11C-Labeled tracers are valuable for studies that demand multiple imaging sessions within the same day, whereas 18F-labeled tracers may be distributed to remote PET imaging facilities that lack an on-site cyclotron and radiochemistry facility. The production of PET tracers for biomedical applications relies on straightforward radiolabeling strategies. For carbon-11, [11C]iodomethane and [11C]methyl triflate are of foremost importance for tracer syntheses through reactions with carbon and heteroatom electrophiles14,15,16,17. For fluorine-18, nucleophilic substitution on appropriate precursors with [18F]fluoride is widely used18,19 (Fig. 1C). However, these and many other methods are limited with regards to the chemotypes that can be labeled and to the possible molecular positions that are open to labeling. Because of tracer metabolism, the position of radiolabel can be a key determinant of PET tracer efficacy20.
There is an ongoing need to expand the range of chemotypes that may be considered as potential PET tracers through the development of labeling synthons and methods21. Methods to label a single tracer with either carbon-11 or fluorine-18 normally require different non-radioactive precursors and entail major synthesis campaigns that may consume excessive time and resources. A more efficient approach is to take advantage of chemical moieties that contain both carbon-11 and fluorine-18 labeling sites and offer labeling via a single precursor. This strategy can simplify the PET radiotracer development process and more effectively address design requirements. In this respect,11C- and 18F-labeled fluoroforms22,23,24 are attractive synthons because they are readily accessible through rapid on-line syntheses as precursors to putatively metabolically stable trifluoromethyl groups (Fig. 1C). Nonetheless, these labeling synthons have been applied almost exclusively to labeling arenes. Application to labeling non-functionalized aliphatic substrates has not been demonstrated.
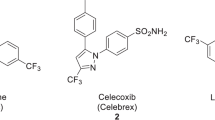
The 2,2,2-trifluoroethoxy group25 has notable metabolic stability26 and moderate lipophilicity and is found in numerous biologically active compounds27,28,29,30. Several PET radiotracers feature a 2,2,2-trifluoroethoxy moiety (e.g., tracers for COX-131,32, tauopathy33,34,35,36, and Huntington aggregates37). Installation of a radiolabeled 2,2,2-trifluoroethoxy group in such tracers has been approached through either nucleophilic addition of [18F]fluoride to a geminal difluorovinyl precursor31,33,36,37 or alkylation of a phenoxy precursor with [18F]2,2,2-trifluoroethoxy tosylate31,35,38. However, both methods suffer from limited substrate scope and low molar activity (ratio of radioactivity to mass of tracer isotopologues) that may make the derived tracers unsuitable for application20,39.
Herein, we describe a rapid and transition metal-free conversion of fluoroform into highly reactive potassium 2,2,2-trifluoroethoxide (CF3CH2OK) and demonstrate robust applications of this synthon in one-pot, two-stage 2,2,2-trifluoroethoxylations of both aromatic and aliphatic precursors (Fig. 1B). Moreover, we show that this transformation translates easily to both carbon-11 and fluorine-18 chemistry, so allowing the appendage of complex molecules with 11C- or 18F- labeled 2,2,2-trifluoroethoxy groups (Fig. 1D). We also exemplify syntheses of isotopologues of potassium 2,2,2-trifluoroethoxide and show their utility for stable isotopic labeling.
Results and discussion
Synthesis and reactivity of potassium 2,2,2-trifluoroethoxide
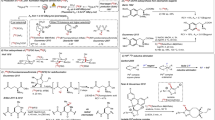
Nucleophilic addition of fluoroform to aldehydes to produce carbinols is well-known40. However, the reaction of fluoroform with the simplest aldehyde, formaldehyde, has not been explored. We considered that this reaction should produce a very useful reagent, 2,2,2-trifluoroethoxide. Because of the toxicity and volatility of formaldehyde41, we decided to explore solid paraformaldehyde as an alternative for producing 2,2,2-trifluoroethoxide. Treatment of fluoroform as limiting reagent with a mixture of paraformaldehyde and t-BuOK in DMF produced 2,2,2-trifluoroethanol in 41% yield after water quench (Supplementary Table 1, entry 1). Under optimal conditions, fluoroform was converted into 2,2,2-trifluoroethanol in 88% yield within 30 min (Supplementary Table 1, entry 13). Increasing the reaction time did not improve the conversion appreciably (Supplementary Table 1, entries 11 and 12). Other bases or solvents adversely affected yield (Supplementary Table 1, entries 14–18). We next tested the reactivity of the putative intermediate, potassium 2,2,2-trifluoroethoxide, by treatment with 2-chloropyrimidine. To our delight, reaction proceeded smoothly at room temperature to afford 2-(2,2,2-trifluoroethoxy)pyrimidine1 in 71% yield within 2 h (Fig. 2A). However, treatment of potassium 2,2,2-trifluoroethoxide with 1-fluoronaphthalene gave only trace 1-(2,2,2-trifluoroethoxy)naphthalene2, even at elevated temperature. We considered that an aryl hypervalent iodine center in an aryliodonium ylide might show more reactivity. Indeed, treatment of potassium 2,2,2-trifluoroethoxide with naphthalen-1-yl iodonium-(2,2-dimethyl-1,3-dioxane-4,6-dione)ylide at 60 °C produced 2 in 63% yield (Fig. 2A). Thus, gratifyingly, we had succeeded in converting stoichiometric amounts of fluoroform into a useful synthon, potassium 2,2,2-trifluoroethoxide, in high yield and in showing the utility of this synthon for trifluoroethoxylation of homoarene and heteroarene under mild conditions. We anticipate that this transition metal-free transformation can find wide applications in fluorine chemistry. Indeed, we readily produced several trifluoroethoxy compounds in high yields by this chemistry as standards for use in the remainder of this study (see Supporting Information, Supplementary methods).
A One-pot two-stage trifluoroethoxylations with fluoroform to give 2,2,2-trifluoroethyl ethers. Yields were determined with 19F NMR spectroscopy. (For more detail, see Supplementary Table 1 and Supplementary Fig. 1). B Optimization of conversion of [11C]fluoroform with paraformaldehyde into [2-11C]2,2,2-trifluoroethanol (left panel) and HPLC chromatogram of the crude reaction mixture from entry 5 (right panel). C Example of one-pot, two-stage synthesis of 2-[11C](2,2,2-trifluoroethoxy)pyrimidine.
Synthesis of [11C]potassium 2,2,2-trifluoroethoxide
We next aimed to explore this synthetic methodology for robust broad-scope radio-trifluoroethoxylations as a potential route to PET tracers. For this purpose, we routinely produce [11C]fluoroform by CoF3-mediated fluorination of cyclotron-produced [11C]methane22. Treatment of [11C]fluoroform (37–296 MBq) with a mixture of t-BuOK (50 μmol) and paraformaldehyde (17 μmol) in DMF for only 3 min at room temperature gave an excellent yield (88%) of 11CF3CH2OH upon hydrolysis of the putative intermediate (Fig. 2B, entry 1). Increasing the reaction time to 5 min only slightly increased the yield. A larger quantity of paraformaldehyde (50 µmol) afforded 11CF3CH2OH in 97% yield. The yield of 11CF3CH2OH was quantitative when [11C]fluoroform was treated with 1: 2 molar mixture of paraformaldehyde and t-BuOK for 4 min (Fig. 2B, entry 5). These reaction conditions were therefore deemed optimal. This success encouraged us to test the efficacy of 11CF3CH2OK for introducing the 11C-trifluoromethyl moiety into a wide range of compounds.
Synthesis of 11C-2,2,2-trifluoroethoxy arenes
First, we examined the reactivity of 11CF3CH2OK towards heteroarenes. To our delight, 11CF3CH2OK reacted at room temperature to produce a wide range of desired 11C-2,2,2-trifluoroethoxy heteroarenes in moderate to excellent yields within just 1 min (Fig. 3A). Attractive features to emerge from this labeling protocol were: (1) the 11CF3CH2OK, can be used without isolation; (2) reaction conditions are mild and rapid; (3) substrate scope is broad, and encompasses pyridines, pyrimidines, pyrazine, thiazoles, triazines, quinolines, and isoquinolines; (4) in addition to halides (F, Cl, and Br), leaving groups such as nitro ([11C]4) and methyl sulfone ([11C]9) are highly effective; (5) functional group tolerance is high, with aldehyde ([11C]3), bromo ([11C]4, [11C]6), nitrile ([11C]5), methoxy ([11C]7), and Boc protection ([11C]9) all well tolerated. Heteroarenes having 1 to 3 nitrogen atoms ([11C]1, [11C]3–[11C]14) were compatible with the reaction conditions and afforded the desired 11C-labeled products in acceptable yields (21–94%). Furthermore, the late-stage 11C-trifluoroethoxylation of complex biomolecules was highly effective as shown by the labeling of analogs of several drug-like compounds [Imiquimod ([11C]15), Milrinone ([11C]18)], drug precursors [Erlotinib ([11C]19), Canagliflozin ([11C]20), Pazopanib ([11C]21), Palbociclib ([11C]22)], the herbicide Clopyralid ([11C]17), and a purine derivative ([11C]16)] in moderate to very good yields. Notably, 11C-trifluoroethoxylation occurred preferentially at the more electron-deficient site (e.g., the ortho- vs meta-pyridinyl site for [11C]17) or aryl ring (e.g., the pyridinyl vs homoarene ring in [11C]6 and [11C]20) as expected for aromatic nucleophilic substitution reactions.
LG = leaving group. Yields are based on HPLC analyses of reaction mixtures. All yields are based on [11C]fluoroform conversion into the products, decay-corrected and expressed as mean ± SD (n = 3). Radioactive products were collected at least once for each substrate to check that HPLC yields matched isolated yields. A Substrate scope for heteroarenes using reaction condition (A). B Substrate scope for homoarenes using reaction condition (B). Auxiliary derived from Meldrum’s acid.
We found that homoarene precursors with common leaving groups were more challenging for 11C-trifluoroethoxylation than the reactive heteroarenes42. We anticipated that the use of a more powerful hypervalent aryliodonium leaving group43,44 could alleviate this issue. We opted to explore this possibility for the one-pot 11C-trifluoroethoxylation of homoarene substrates. First, we screened conditions for the reaction of [11C]potassium 2,2,2-trifluoroethoxide with an iodonium ylide derived from Meldrum’s acid (naphthalen-1-yl(2,4,6-trimethoxyphenyl)iodonium tosylate) (Supplementary Table 3). We found that treating a mixture of [11C]CF3CH2OK with the iodonium salt precursor (55 µmol) in DMF at 60 °C for 3 min gave a high and optimal yield of the desired [11C]2 (87%; Supplementary Table 3, entry 2). The 2,4,6-trimethoxyphenyl group served as an effective aryl spectator ring; no [11C]1,3,5-trimethoxy-(2-(2,2,2-trifluoroethoxy))benzene, was produced. An increase in temperature did not improve the yield of [11C]2. Reduction in precursor amount reduced yield. Given the high yield obtained for [11C]2 with this approach under optimal conditions, we proceeded to explore substrate scope. Substituent electronics had substantial influence on reaction yields ([11C]23–[11C]32). Electron-withdrawing groups in ortho- and para-position gave high yields for the 11C-trifluoroethoxylation (Fig. 3B). 11C-Trifluorethoxylation yields were lower for substrates with para-electron-donating or meta-electron-withdrawing groups. Novel cross-coupling synthons, [11C]24–[11C]27, were obtained in useful yields. We draw attention to the syntheses of [11C]26 and [11C]28, where mesityl was used as the partner aryl ring in the iodonium salt precursor11.C-Trifluoroethoxylation was directed to the other aryl ring. This is an interesting observation because here the ring chemoselectivity is opposite to that seen for the non-copper mediated radiofluorination of aryl(mesityl)iodonium salts45.
We also explored aryliodonium ylides as precursors. 11C-Trifluoroethoxylation of three model ylides gave [11C]33–[11C]35, in moderate to high yields. Moreover, [11C]36, an analog of the antidiabetic drug emplagliflozin (®Jardiance) was also obtained in moderate yield (47%) from an ylide. This exemplifies how iodonium ylides can serve as precursors for trifluoroethoxylation reactions. In addition, two activated fluoroarenes, with ortho-electron-deficient aryl rings, gave [11C]37 and [11C]38 in moderate to good yields where fluoride was the leaving group.
11C-2,2,2-Trifluoroethoxylation of aliphatic substrates
We were further interested in whether 11C-2,2,2-trifluoroethoxylation would occur on aliphatic substrates as well as arenes. This consideration prompted us to investigate the reactivity of 11CF3CH2OK with aliphatic substrates. We started with a model compound, a precursor to Posaconazole (®Noxafil) with a tosylate leaving group to optimize precursor amount and reaction temperature (Supplementary Table 4). 11C-Trifluoroethoxylation produced excellent yields of [11C]45 (89%) under conditions found to be optimal for hypervalent iodonium precursors (Supplementary Table 4, entry 3). Again, yield did not increase with temperature (Supplementary Table 4, entry 4). Reduction in precursor amount drastically diminished yield (Supplementary Table 4, entries 5 and 6). We next tested reaction scope by attempting to prepare a range of 11C-labeled alkyl-2,2,2-trifluoroethyl ethers from aliphatic precursors, including fourteen 11C-labeled biomolecules (Fig. 4). [11C]41 was obtained from a long chain iodoalkyl precursor in acceptable yield (45%). Benzyl halide and α-chloroacetyl precursors were readily converted into their analogous 11C-2,2,2-trifluoroethoxy ethers ([11C]42, [11C]47, [11C]48, [11C]51, [11C]52) in high yields (53–89%) with good tolerance of other functional groups. Aliphatic tosylates derived from a variety of commercially available drug-like molecules MCPA (2-methyl-4-chlorophenoxyacetic acid), Helional, Ketoconazole, Bendazac, an α-D-glucopyranoside derivative, Oxaprozin, Ospemifene, Pterostilbene, and Cyhalofop-butyl reacted readily with 11CF3CH2OK to provide the desired 11C-2,2,2-trifluoroethoxy ethers, [11C]39, [11C]40, [11C]43–[11C]46, [11C]49, [11C]50, and [11C]53–[11C]56, in moderate to excellent yields (34–93%). Precursors with leaving groups attached to an ethylene glycol linker gave excellent yields of 11C-2,2,2-trifluoroethoxy ethers. Notably, aliphatic 11C-trifluoroethoxylation occurred in preference to reaction at aromatic sites.
LG = leaving group, Decay-corrected yields are based on HPLC analyses of crude reaction mixtures. All yields are based on [11C]fluoroform conversion into the products and expressed as mean ± SD (n = 3). Radioactive products were collected at least once for each substrate to check that HPLC yields matched isolated yields.
Determination of molar activity of [11C]1 as a model compound
We measured the molar activity for a model product [11C]1, produced by the 11C-trifluoroethoxylation of 2-chloropyrimidine, to verify that this labeling technique is no-carrier-added (NCA) and gives high molar activity. Starting with about 10 GBq of cyclotron-produced [11C]methane that has been produced from a 10 µA × 10 min cyclotron irradiation, [11C]1 was obtained with a molar activity of 60 GBq/µmol, corrected to the end of radionuclide production (ERP). Such a high molar activity from a relatively limited cyclotron irradiation shows that the labeling reaction is invulnerable to carrier addition and dilution of molar activity15,39.
Synthesis of [18F]potassium 2,2,2-trifluoroethoxide
Fluorine-18 labeling of PET tracers at aliphatic carbon by nucleophilic substitution of a good leaving group with [18F]fluoride19 can often lead to an [18F]fluoroalkyl group that is vulnerable to radiodefluorination in vivo and to accumulation of [18F]fluoride ion in the bone including skull. This can hamper accurate quantification of tracer uptake, especially in brain20,46,47.18F-Labeling in a 2,2,2-trifluoroethoxy group instead of an 18F-fluoroalkyl group could be a strategy to circumvent this issue. Having established an efficient route for 11C-trifluoroethoxylation, we next focused on translation of this labeling method from carbon-11 to fluorine-18 with a few representative substrates. For this purpose, [18F]fluoroform was produced from no-carrier-added [18F]fluoride and difluoroiodomethane48. CF218FCH2OK was generated by treatment of the [18F]fluoroform with paraformaldehyde and t-BuOK in DMF in >95% yield. The reactivity of CF218FCH2OK was assessed under the optimal conditions found for 11C-trifluoroethoxylations (Fig. 5).18F-Trifluoroethoxylations of aryl and aliphatic precursors proceeded smoothly and provided corresponding products in moderate to excellent yields similar to those from 11C-trifluoroethoxylation. Heteroarenes, such as pyridine, quinoline, pyrimidine, isothiazole, and 1,3,5-triazine, with halogen leaving groups were converted into the corresponding 18F-2,2,2-trifluoroethoxy ethers in high yields (82–96%; Fig. 5A). Dependency of labeling position on aryl ring position of the leaving group (e.g., ortho- vs meta- as in [18F]4 and [18F]17) or on the nature of the aryl ring (e.g., [18F]20) was as seen for 11C-labeling. Heteroaryl rings with more structural complexity and diverse functionality were conveniently labeled at room temperature within 5 min and produced the corresponding 18F-labeled compounds in excellent yields ([18F]15, [18F]17–[18F]22; 56–77%; Fig. 5A). These results indicate high potential for application of this labeling method to prospective structurally complex PET tracers. Homoarene precursors, including diaryliodonium salts ([18F]27 and [18F]31), aryliodonium ylides ([18F]35 and [18F]36), and fluoro precursors ([18F]37 and [18F]38), afforded useful yields of 18F-labeled products (27–69%; Fig. 5B), as for the11C-trifluoroethoxylations. Remarkably, the unprotected hydroxyl group in the Ataluren precursor was well tolerated ([18F]38) showing compatibility of this labeling protocol to sensitive functionality.
LG = leaving group. Yields are calculated from HPLC analyses of crude reaction mixtures and based are [18F]fluoroform conversion into the products. Radioactive products were collected at least once for each substrate to confirm that HPLC yields match isolated yields. All yields are decay-corrected and reported as mean ± SD for n = 3. A Substrate scope for homoarenes using reaction condition (B). B Substrate scope for heteroarenes using reaction condition (B). C Substrate scope for aliphatic compounds using reaction condition (B).
Furthermore, we were keen to know whether potassium 18F-2,2,2-trifluoroethoxide could be useful for labeling at aliphatic carbon, given the limited availability of methods for constructing stable alkyl-CF218F bonds49. In this regard, we tested identical reaction conditions to those used for 11C-trifluoroethoxylation on diverse aliphatic substrates, prepared from drugs, herbicides, and other biomolecules. To our delight, this protocol successfully enabled the installation of a 18F-2,2,2-trifluoroethoxy moiety onto aliphatic carbon in a variety of complex structures in acceptable to excellent yields ([18F]44–[18F]46, [18F]49–[18F]56; 15–95%; Fig. 5C). Taken together, these results show that this methodology, based on the transformation of fluoroform into potassium 2,2,2-trifluoroethoxide and subsequent functionalization of aliphatic carbons, is equally versatile for both carbon-11 and fluorine-18 with the same non-radioactive precursor.
Determination of molar activity of [18F]1 as a model compound
We measured the molar activity of [18F]1 to be 1.3 GBq/µmol, decay-corrected. The method of [18F]fluoroform synthesis that we used was one reported in the literature48 and known to give low molar activity. We did not observe any significant release of fluoride ion in the production of [18F]potassium 2,2,2-trifluoroethoxide under basic conditions, as evidenced by absence of [18F]fluoride at the solvent front in the HPLC analysis of derived [18F]2,2,2-trifluoroethanol (Supplementary Fig. 17). Therefore, the molar activity of the starting [18F]fluoroform determines the molar activity of 18F-labeled 2,2,2-trifluoroethoxy products.
Synthesis of isotopologues of potassium 2,2,2-trifluoroethoxide
Isotopologues differ only in their isotopic substitutions and play an important role in drug development50. Deuteration51 is widely practiced to improve the metabolic stability of PET tracers in 18F-fluoroalkyl positions.13C-Labeling enables investigations of drug pharmacokinetics and metabolism by 13C-NMR spectroscopy and mass spectrometry52,53. Simple methods for accessing stable isotopically labeled compounds are highly desirable. The availability of isotopically labeled fluoroforms (H13CF3, H11CF3, and HCF218F) and paraformaldehyde [(CD2O)n and (13CH2O)n] and our method for the in situ generation of CF3CH2OK from fluoroform, provide an opportunity to explore the incorporation of isotopically labeled 2,2,2-trifluoroethoxy groups into a diverse array of substrates. For demonstration, we synthesized isotopologues of 57, an analog of a well-known COX-1 PET tracer, [11C]PS1332. Compounds [11C]57 and [18F]57 were readily obtained by treating a tosylate precursor with [11C/18F]CF3CH2OK under optimized conditions. The reaction was equally effective when substituting paraformaldehyde with (CD2O)n and (13CH2O)n, leading to high yield syntheses of [2H]57, [2H/11C]57, [2H/18F]57, [13C]57, [13C/11C]57, and [13C/18F]57 (Fig. 6). Hence, this isotope labeling protocol has exceptional potential for broad application.
In summary, based on the transformation of paraformaldehyde with fluoroform, we devised a highly effective one-pot method for appending a wide range of aryl, heteroaryl, and aliphatic organic compounds with an isotopically labeled 2,2,2-trifluoroethoxy group. Especially, reaction of paraformaldehyde with [11C]fluoroform or [18F]fluoroform efficiently provides 11CF3CH2OK and 18FF2CCH2OK, respectively, as broadly useful no-carrier-added labeling synthons with ability to produce candidate PET tracers bearing either a 11C- or 18F-labeled 2,2,2-trifluoroethoxy group. Use of paraformaldehyde and fluoroform labeled with stable isotopes (2H, or 13C) gives ready access to isotopologues of 2,2,2-trifluoroethoxy compounds. Consequently, the 2,2,2-trifluoroethoxy group may garner increasing interest for both drug and PET tracer development.
Methods
General procedure for the one-pot 2,2,2-trifluoroethoxylation of aromatic and aliphatic precursors from fluoroform and isotopically labeled paraformaldehyde
Isotopically labeled (13C and 2H) paraformaldehyde (3.0 equiv.) and t-BuOK (3.0 equiv.) were added to a round-bottomed flask equipped with a magnetic stirrer bar followed by DMF (6 mL/1 mmol) under argon atmosphere. A solution of fluoroform (1.0 equiv., 0.3 M) in DMF was added and the reaction mixture was stirred at RT for 3 h. Precursor solution in DMF was added, and the reaction mixture was stirred at RT or 60 °C for additional 2 h. The reaction was then quenched with water (2 mL) and the mixture was extracted with DCM (2 × 10 mL). The organic phase was washed with brine, dried (Mg2SO4), and concentrated under reduced pressure. The residue was purified by flash chromatography on silica gel to give the 2,2,2-trifluoroethyl ether. Purification methods and characterization data of all the individual trifluoroethoxy compounds synthesized in this work can be obtained in the Supplementary Methods Section 1.2, 1.3 and 2.4.
Synthesis of [11C]Fluoroform22
[11C]Methane (3.7–5.5 GBq) was produced from a cyclotron (PETtrace; GE Healthcare) according to the 14N(p.α)11C reaction by irradiating nitrogen (130 psi) containing hydrogen (7%) and then trapped in a U-tube packed with Porapak Q (1 g, 80–100 mesh) that was being cooled with liquid argon. Any untrapped radioactivity was captured in a waste bag for safety. When radioactivity in the cooled Porapak Q trap had maximized, as indicated by a nearby radiation detector, the liquid argon coolant was removed. The trap was then allowed to warm to room temperature while being purged with a controlled flow of helium (20 mL/min) to direct and pass the [11C]methane sequentially through a Sicapent column (to remove any moisture), a CoF3 column heated at 270 °C, a cooled trap for HF, and finally into a [11C]fluoroform collection trap being cooled in liquid argon. This transfer generally took about 15 min. Then the [11C]fluoroform trap was placed in a warm water bath (60 °C) for another 35–45 s to release [11C]fluoroform into a vial containing DMF (0.8 mL) at –40 °C. The yield of [11C]fluoroform from [11C]methane was usually 35–55% accompanied by 10–50% [11C]fluoromethane (Supplementary Fig. 12). This [11C]fluoroform solution in DMF was used for further reactions. The percentage of radioactivity represented by [11C]fluoroform in the HPLC analyte was calculated from the radio-HPLC chromatogram from peak areas with a correction for radioactive decay between radioactive peaks during the analysis. The yields of the reaction products are based on the conversion of [11C]fluoroform into radioactive products and are calculated by decay-correction to the beginning of the HPLC analysis. We confirmed that all the radioactivity injected onto the HPLC column was fully recovered.
Synthesis of [18F]Fluoroform48
[18F]Fluoride ion was produced on a cyclotron (PETtrace; GE Healthcare) according to the 18O(p,n)18F reaction by irradiating 18O-enriched water (3 mL, 98 atom%) with a beam of protons (16.5 MeV; 35–45 µA) for at least 75 min. [18F]Fluoroform was synthesized within a lead-shielded hot-cell with a fully automated apparatus (TRACERlabTM FX2N; GE Healthcare). Thus, [18F]fluoride ion (3.72–11.1 GBq) in [18O]water (200–400 µL) and a solution (100 µL) containing K2CO3 (3.4 µmol) plus K 2.2.2 (13.6 µmol) were loaded into a glass vial. MeCN (2 mL) was added, and the solvent was azeotropically removed at 80‒100 °C under a stream of nitrogen gas that was vented to vacuum. This step was repeated after a second addition of MeCN (2 mL). A solution of difluoroiodomethane (8.0 mg, 45 µmol) in anhydrous acetonitrile (1.0 mL) was then added to the dried [18F]fluoride-K2CO3/K 2.2.2 complex, sealed, and heated at 35 °C for 10 min. [18F]Fluoroform was flushed out of the vial with helium (20 mL/min) and into the [18F]fluoroform trap. The transfer generally required 5 min. Then the [18F]fluoroform trap was put in warm water bath (60 °C) for another 35–45 s to release [18F]fluoroform into a vial containing DMF (0.8 mL). This DMF solution of [18F]fluoroform was used for subsequent reactions. The yield of [18F]fluoroform produced by this method generally ranged between 35–65% from dried [18F]fluoride with >98% radiochemical purity (Supplementary Fig. 15).
General procedure for the 11C- and 18F- 2,2,2-trifluoroethoxylation of aromatic and aliphatic precursors from isotopically labeled fluoroform and paraformaldehyde
About 30 min before the end of radionuclide production, a mixture of t-BuOK (112.2 mg, 1.0 mmol) and paraformaldehyde (15.0 mg, 0.5 mmol) in DMF (6 mL) was prepared in a glovebox under argon and kept there until close to the start of radiochemistry. The t-BuOK-paraformaldehyde reagent mixture in DMF (200 µL) was added to a 1-mL V vial, septum-sealed, removed from the glovebox, and transferred to the lead-shielded hot-cell for the radiochemistry. [11C]Fluoroform or [18F]fluoroform (37–296 MBq) in DMF (50–300 μL) was added to the vial, mixed, and left at RT for 4 min. A DMF solution (200 µL) of the aromatic or aliphatic precursor (55 μmol) was then added. The reaction mixture was kept at RT for 1 min or heated at 60 °C for 3 min and then quenched with water (100 μL). This crude product was analyzed with radio-HPLC as detailed in Supplementary Methods section. Areas of all the radiochemical product peaks were decay-corrected to the beginning of the HPLC analysis for radiochemical yields calculations. HPLC analysis of each 11C- and 18F- labeled trifluoroethoxylated products and the respective HPLC chromatograms can be obtained in Supplementary Methods Sections 6 and 7.
Data availability
Details about materials and methods, experimental procedures including organic syntheses and radiochemistry, and NMR spectra and HPLC chromatographs are available in the Supplementary Information. Any further queries on the data can be directed to either S.T or V.W.P.
References
Li, H. P., He, X. H., Peng, C., Li, J. L. & Han, B. A straightforward access to trifluoromethylated natural products through late-stage functionalization. Nat. Prod. Rep. 40, 988–1021 (2023).
Charpentier, J., Früh, N. & Togni, A. Electrophilic trifluoromethylation by use of hypervalent iodine reagents. Chem. Rev. 115, 650–682 (2015).
Liu, X., Xu, C., Wang, M. & Liu, Q. Trifluoromethyltrimethylsilane: Nucleophilic trifluoromethylation and beyond. Chem. Rev. 115, 683–730 (2015).
Xiao, H. W., Zhang, Z. Z., Fang, Y. W., Zhu, L. & Li, C. Z. Radical trifluoromethylation. Chem. Soc. Rev. 50, 6308–6319 (2021).
Rozen, S. & Hagooly, A. in e-EROS Encyclopedia of Reagents for Organic Synthesis 1‒3 (John Wiley & Sons, Ltd., 2001).
Luo, Z., Cahard, D. & Tsui, G. C. Using fluoroform in trifluoromethylation reactions. J. Fluorine Chem. 266, 110092 (2023).
Russell, J. & Roques, N. Effective nucleophilic trifluoromethylation with fluoroform and common base. Tetrahedron 54, 13771‒13782 (1998).
Prakash, G. K. S., Jog, P. V., Batamack, P. T. D. & Olah, G. A. Taming of fluoroform: direct nucleophilic trifluoromethylation of Si, B, S, and C Centers. Science 338, 1324–1327 (2012).
Tomashenko, O. A. & Grushin, V. V. Aromatic trifluoromethylation with metal complexes. Chem. Rev. 111, 4475–4521 (2011).
Zanardi, A., Novikov, M. A., Martin, E., Benet-Buchholz, J. & Grushin, V. V. Direct cupration of fluoroform. J. Am. Chem. Soc. 133, 20901–20913 (2011).
Xiang, J. X., Ouyang, Y., Xu, X. H. & Qing, F. L. Argentination of fluoroform: preparation of a stable AgCF3 solution with diverse reactivities. Angew. Chem. Int. Ed. 58, 10320–10324 (2019).
Phelps, M. E. Positron emission tomography provides molecular imaging of biological processes. Proc. Natl. Acad. Sci. 97, 9226–9233 (2000).
Cherry, S. R. et al. Total-body imaging: transforming the role of positron emission tomography. Sci. Transl. Med. 9, eaaf6169 (2017).
Ametamey, S. M., Honer, M. & Schubiger, P. A. Molecular imaging with PET. Chem. Rev. 108, 1501–1516 (2008).
Pike, V. W. Considerations in the development of reversibly binding PET radioligands for brain imaging. Curr. Med. Chem. 23, 1818–1869 (2016).
Rong, J., Haider, A., Jeppesen, T. E., Josephson, L. & Liang, S. H. Radiochemistry for positron emission tomography. Nat. Commun. 14, 3257 (2023).
Wuest, F., Berndt, M. & Kniess, T. in PET Chemistry—The Driving Force in Molecular Imaging (eds. P. A. Schubiger, L. Lehmann & M. Friebe) 183–213 (Springer, 2007).
Preshlock, S., Tredwell, M. & Gouverneur, V. 18F-Labeling of arenes and heteroarenes for applications in positron emission tomography. Chem. Rev. 116, 719–766 (2016).
Siméon, F. G., Telu, S., Cai, L. S., Lu, S. & Pike, V. W. in The Chemistry of Organofluorine Compounds, Patai's Chemistry of Functional Groups 1st edn (eds. I. Marek, V. Gouverneur, & M. Gandelman) 640 (John Wiley & Sons Ltd, 2023).
Pike, V. W. PET radiotracers: crossing the blood–brain barrier and surviving metabolism. Trends Pharmacol. Sci. 30, 431–440 (2009).
Honer, M., Gobbi, M., Martarello, L. & Comley, R. A. Radioligand development for molecular imaging of the central nervous system with positron emission tomography. Drug Discov. Today 19, 1936–1944 (2014).
Haskali, M. B. & Pike, V. W. [11C]Fluoroform, a breakthrough for versatile labeling of PET radiotracer trifluoromethyl groups in high molar activity. Chem. Eur. J. 23, 8156–8160 (2017).
Pees, A., Windhorst, A. D., Vosjan, M. J. W. D., Tadino, V. & Vugts, D. J. Synthesis of [18F]fluoroform with high molar activity. Eur. J. Org. Chem. 2020, 1177–1185 (2020).
Chai, J. Y. et al. Mechanistic study of nucleophilic fluorination for the synthesis of fluorine-18 labeled fluoroform with high molar activity from N-difluoromethyltriazolium triflate. RSC Adv. 11, 6099–6106 (2021).
Pethő, B. & Novák, Z. Synthesis of aryl- and heteroaryl-trifluoroethyl ethers: aims, challenges and new methodologies. Asian J. Org. Chem. 8, 568–575 (2019).
Irurre, J. Jr., Casas, J. & Messeguer, A. Resistance of the 2,2,2-trifluoroethoxy aryl moiety to the cytochrome P-450 metabolism in rat liver microsomes. Bioorg. Med. Chem. Lett. 3, 179–182 (1993).
Kawamura, K., Morita, M. & Yamagishi, T. Pyrrolopyridinone derivatives as TTX-S blockers. PCT Int. Appl. WO2013161312A1 (2013). https://patents.google.com/patent/WO2013161312A1.
Nakada, Y. et al. Novel acyl coenzyme A (CoA): diacylglycerol acyltransferase-1 inhibitors: synthesis and biological activities of diacylethylenediamine derivatives. Bioorg. Med. Chem. 18, 2785–2795 (2010).
Slemon, C. & Macel, B. Preparation of omeprazole and lansoprazole and intermediates useful therein. PCT Int. Appl. WO1995012590A1 (1995). https://patents.google.com/patent/WO1995012590A1.
Yoshida, M., Homma, Y. & Kawabe, K. Silodosin, a novel selective α1A-adrenoceptor selective antagonist for the treatment of benign prostatic hyperplasia. Expert Opin. Investig. Drugs 16, 1955–1965 (2007).
Taddei, C. et al. Synthesis of [18F]PS13 and evaluation as a PET radioligand for cyclooxygenase-1 in monkey. ACS Chem. Neurosci. 12, 517–530 (2021).
Singh, P. et al. 3‑Substituted 1,5-diaryl‑1H‑1,2,4-triazoles as prospective PET radioligands for imaging brain COX‑1 in monkey. Part 1: synthesis and pharmacology. ACS Chem. Neurosci. 9, 2610–2619 (2018).
Kramer, V. et al. Evaluation of [18F]-N-methyl lansoprazole as a tau PET imaging agent in first-in-human studies. ACS Chem. Neurosci. 11, 427–435 (2020).
Rafique, W. et al. Image-guided development of heterocyclic sulfoxides as ligands for tau neurofibrillary tangles: from first-in-man to second generation ligands. ACS Omega 3, 7567–7579 (2018).
Riss, P. J. et al. Radiosynthesis and characterization of astemizole derivatives as lead compounds toward PET imaging of τ-pathology. MedChemComm. 4, 852–855 (2013).
Fawaz, M. V. et al. High affinity radiopharmaceuticals based upon lansoprazole for PET imaging of aggregated tau in alzheimer’s disease and progressive supranuclear palsy: synthesis, preclinical evaluation, and lead selection. ACS Chem. Neurosci. 5, 718–730 (2014).
Kaur, T. et al. Synthesis and evaluation of a fluorine-18 radioligand for imaging huntingtin aggregates by positron emission tomography. Front. Neurosci. 15, 766176 (2021).
Francis, F. & Wuest, F. Advances in [18F]trifluoromethylation chemistry for PET imaging. Molecules 26, 6478 (2021).
Luurtsema, G. et al. EANM guideline for harmonization on molar activity or specific activity of radiopharmaceuticals: impact on safety and imaging quality. EJNMMI Radiopharm. Chem. 6, 34 (2021).
Shono, T., Ishifune, M., Okada, T. & Kashimura, S. Electroorganic chemistry. 130. A novel trifluoromethylation of aldehydes and ketones. J. Org. Chem. 56, 2–4 (1991).
Morgan, K. T. A brief review of formaldehyde carcinogenesis in relation to rat nasal pathology and human health risk assessment. Toxicol. Pathol. 25, 291–307 (1997).
Rohrbach, S., Murphy, J. A. & Tuttle, T. Computational study on the boundary between the concerted and stepwise mechanism of bimolecular SNAr reactions. J. Am. Chem. Soc. 142, 14871–14876 (2020).
Okuyama, T., Tomoki Takino, T., Sueda, T. & Ochiai, M. Solvolysis of cyclohexenyliodonium salt, a new precursor for the vinyl cation: remarkable nucleofugality of the phenyliodonio group and evidence for Internal return from an intimate ion‒molecule pair. J. Am. Chem. Soc. 117, 3360–3367 (1995).
Pike, V. W. Hypervalent aryliodine compounds as precursors for radiofluorination. J. Label. Compd. Radiopharm. 61, 196–227 (2018).
Chun, J. ‐H., Lu, S., Lee, Y. ‐S. & Pike, V. W. Fast and high‐yield micro‐reactor syntheses of ortho‐substituted [18F]fluoroarenes from [18F]fluoride ion with diaryliodonium salts. J. Org. Chem. 75, 3332–3338 (2010).
Park, B. K. & Kitteringham, N. R. Effects of fluorine substitution on drug metabolism: pharmacological and toxicological implications. Drug Metab. Rev. 26, 605–643 (1994).
Kuchar, M. & Mamat, C. Methods to increase the metabolic stability of 18F-radiotracers. Molecules 20, 16186–16220 (2015).
van der Born, D. et al. A universal procedure for the [18F]trifluoromethylation of aryl iodides and aryl boronic acids with highly improved specific activity. Angew. Chem. Int. Ed. 53, 11046–11050 (2014).
Cheguillaume, A., Gillart, J., Labar, D., Grégoire, V. & Marchand-Brynaert, J. Perfluorinated markers for hypoxia detection. Synthesis of sulfur containing precursor and [18F]-labelling. Bioorg. Med. Chem. 13, 1357–1367 (2005).
Bluck, L. & Volmer, D. A. The role of naturally occurring stable isotopes in mass spectrometry, part I: the theory. Spectroscopy (Springf) 24, 34 (2009).
Di Martino, R. M. C., Maxwell, B. D. & Pirali, T. Deuterium in drug discovery: progress, opportunities, and challenges. Nat. Rev. Drug Discov. 22, 562–584 (2023).
Otte, D. A. L., Borchmann, D. E., Lin, C., Weck, M. & Woerpel, K. A. 13C NMR spectroscopy for the quantitative determination of compound ratios and polymer end groups. Org. Lett. 16, 1566–1569 (2014).
Fan, T. W.-M. et al. Stable isotope-resolved metabolomics, and applications for drug development. Pharmacol. Ther. 133, 366–391 (2012).
Acknowledgements
We acknowledge the Intramural Research Program of the National Institutes of Health (NIMH; ZIA-MH002793) for financial support and thank the NIH Clinical Center (Chief Dr. P. Herscovitch) for radioisotope production.
Author information
Authors and Affiliations
Contributions
Q.Z., S.T., S.J., C.L.M., contributed to study design and experimental implementation. S.T. and V.W.P. contributed to study design and supervision. All authors contribute to writing and review of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Junbin Han and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, Q., Telu, S., Jana, S. et al. Isotopologues of potassium 2,2,2-trifluoroethoxide for applications in positron emission tomography and beyond. Nat Commun 15, 5798 (2024). https://doi.org/10.1038/s41467-024-49975-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-49975-7
- Springer Nature Limited