Abstract
Climate warming is one of the facets of anthropogenic global change predicted to increase in the future, its magnitude depending on present-day decisions. The north Atlantic and Arctic Oceans are already undergoing community changes, with warmer-water species expanding northwards, and colder-water species retracting. However, the future extent and implications of these shifts remain unclear. Here, we fitted a joint species distribution model to occurrence data of 107, and biomass data of 61 marine fish species from 16,345 fishery independent trawls sampled between 2004 and 2022 in the northeast Atlantic Ocean, including the Barents Sea. We project overall increases in richness and declines in relative dominance in the community, and generalised increases in species’ ranges and biomass across three different future scenarios in 2050 and 2100. The projected decline of capelin and the practical extirpation of polar cod from the system, the two most abundant species in the Barents Sea, drove an overall reduction in fish biomass at Arctic latitudes that is not replaced by expanding species. Furthermore, our projections suggest that Arctic demersal fish will be at high risk of extinction by the end of the century if no climate refugia is available at eastern latitudes.
Similar content being viewed by others
Introduction
Climate warming is driving changes in the distribution of many species. Expanding ranges towards higher latitudes and contracting ranges in lower latitudes have been widely reported, and are resulting in species richness shifts1,2,3. These distributional shifts are driven by local climate velocities, which often differ from place to place, and do not strictly follow the global patterns of temperature, both in direction and magnitude of change4. The Arctic, warming almost four times faster than the global average5, is experiencing increases of species richness due to the expansion of several warmer-water species, and the contraction of fewer colder-water species; and these changes are expected to continue in the future6,7,8.
Marine fish distribution shifts have significant implications for ecosystems and human activities, particularly for the fishing industry9,10,11, and could result in transboundary conflicts due to the redistribution of commercially important fish species worldwide12,13,14. For all fish species, including non-commercial species, conservation efforts may be challenged by the climate-induced displacement of populations from marine protected areas, or by ecosystem-wide changes derived from species geographic range shifts effects on species interactions, predator-prey dynamics, or food webs15,16,17. In the last decades, SDM projections into the future have provided relevant insights to policy makers, fisheries and conservation managers18,19,20. However, future projections of fish distributions to date either (1) do not include species’ relative abundance or biomass, (2) model species independently, and/or (3) focus on few, common species, often limited to such of interest for fisheries with sufficient data to run species distribution models (SDM).
To overcome these limitations, we applied a joint species distribution model (J-SDM) of 107 Northeast Atlantic marine fish distributions along the continental shelf from the North Sea to the Barents Sea (61 of them including their biomass distribution). We analysed richness and relative dominance trends in Arctic communities with potential changes in species distributions and biomass (as relative abundance) under different future scenarios and investigated the influence of species traits on these future distributions.
The recent development of J-SDMs, and particularly of the Hierarchical Modelling of Species Communities (HMSC) framework, represents an advance over traditional SDMs, and is able to partially overcome the listed limitations by assuming a joint response of species to the environment and to each other21,22. This allows rare species to «borrow» niche information from more common species, particularly from those closely related phylogenetically. Moreover, JSDMs account for co-occurrence patterns betwen species by using latent variables. Although the interpretation of these correlation patterns into biotic interactions cannot be made easily23,24, accounting for them may provide better estimation of the environmental parameters of the model25. For all this, JSDM-HMSC has proven to be among the best predictive statistical distribution models for species communities, particularly in the presence of several rare species22,25.
In the Northeast Atlantic and Arctic Barents Sea, rising temperatures have already led to altered ocean circulation patterns, a decrease in sea ice cover, and profound changes to the marine ecosystems26. In the North Sea, widespread northward displacements have been documented in the planktonic community, in the pelagic and demersal fish communities, and are expected in benthic communities27,28,29. Similarly, the Barents Sea has experienced an arrival of boreal species and a decline of Arctic species, which have their trailing edge within the Barents Sea30,31, leading to compositional changes in the Barents Sea communities, including increases in species richness30,32. Moreover, Arctic species are shifting their biomass centroids northwards at a higher rate than boreal species8. Although these changes are expected to continue in the future6,7, their extent, and their implications for the biomass of future communities remain little investigated. For example, it is unclear to what degree Arctic species will retract or fully disappear from the Barents Sea with climate warming. Understanding the implications of expected range shifts is of critical significance for Arctic communities, given the Arctic’s accelerated warming, the associated higher extinction risk of polar species, and the inherent limitation of Arctic demersal fishes to shift into northern latitudes due to the absence of a contiguous continental shelf5,33,34.
Here we present a JSDM model of the boreal and arctic marine fish communities from the North Sea to the Barents Sea (Fig. 1), and we show overall increases in richness and declines in relative dominance in the community with projected future conditions, as well as generalised increases in species’ ranges and abundance. This comes at the cost of severe declines of Arctic species. Furthermore, the practical disappearance of the two most common fish species in the Barents Sea, namely capelin and polar cod, results in an overall reduction in fish biomass. We predict that Arctic demersal fish species will be at high risk of extinction in the next decades if no climate refugia is available at eastern latitudes.
Results
Environmental correlations
Among selected environmental variables, depth was the best predictive variable in both the presence-absence and biomass models, with an average of 58% and 49% of deviance explained, respectively, followed by sea bottom temperature with 32% and 19% respectively, and the spatial random effect, which accounted for 8% and 29% respectively. Each of the other variables explained on average less than 1% of the total deviance in both models, although this varied by species (Supplementary Fig. 1). For example, phytoplankton concentration explained 20% of variance in the thorny skate (Amblyraja radiata) probability of occurrence, and sea ice concentration explained 15% of variance in herring (Clupea harengus) CPUE distribution.
We found strong support for phylogenetic niche conservatism, with a phylogenetic correlation parameter rho of 0.58, 95% CI [0.41,0.73] in the presence-absence model, and 0.96, 95% CI [0.89,0.95] in the CPUE model, which strongly suggests that species niches in the community are highly determined by phylogenetically structured traits. After accounting for the fixed effects, representing species responses to the environment conditioned on their traits, we found pronounced residual species co-occurrence patterns with strong statistical support (p < 0.05, Supplementary Figs. 2 and 3), although without obvious community patterns.
Species richness and relative dominance
Species richness was projected to increase in the study area across future scenarios (Fig. 2). The biggest increases were projected in the northern Barents Sea, with doubling of species richness around Svalbard and the north coast of Norway. No increases, and even slight decreases in richness were projected in the deepest part of the Barents Sea, at the Bear Island Trench (Fig. 2A). Smaller increases in species richness were projected in the centre of the North Sea and small declines elsewhere in the North Sea and southern Norwegian Sea.
Relative dominance (%) was weakly inversely correlated with species richness (Pearson = −0.09, p < 0.01) and accordingly, we projected declines in percentage of relative dominance in the northern and eastern Barents Sea (Fig. 2B). Species relative dominance changed abruptly for polar cod (Boreogadus saida), which went from dominating in 29% of the study area under present-day conditions, to a lack of dominance in any grid cell in the study area under the high emission pathway in 2100 (Table 1). The species that increased most notably their relative dominance in accordance were Norway pout (Trisopterus esmarkii), blue whiting (Micromesistius poutassou), and whiting (Merlangius merlangus) (Table 1).
Individual species geographic range shifts
Species were generally projected to increase their distribution range and biomass with high emission scenarios, particularly in their core range (Fig. 3, Supplementary Data 1). However, this was not a homogeneous response, and differences between contracting and expanding species increased under the high emission scenarios (Figs. 3 and 4 and Supplementary Figs. 4 and 5). The few Arctic species for which biomass models were considered adequate (n = 3), and the Arctic-boreal species (n = 8) were projected to strongly decline across all scenarios, while boreal (n = 40) and warmer-water species (n = 5) were projected to expand across the whole study area (Fig. 4). However, present-day projected biomass of currently abundant species (mostly polar cod Boreogadus saida, and capelin Mallotus villosus) was not compensated by expanding boreal species in future scenarios, leading to an overall decline in biomass with climate warming (Fig. 5).
Projected rate of change in A species’ geographic range change, B species’ biomass, C species’ core range, and D species’ core biomass, at each socioeconomic pathway between 2010 and 2100. Boxplots are a standardized way of displaying the distribution of the data by showing the median percentage change of all species (black line) while box limits correspond to the interquantile range (IQR). Whiskers correspond to maximum and minimum values, calculated as Q3 + 1.5xIQR and Q1 – 1.5xIQR, and points correspond to outliers. Asteriscs indicate signifiant difference from 0 (Two sided Wilcoxon rank test, p < 0.05, see Source Data for individual test results). Biomass units are in catch per unit effort (CPUE fish/min).
Mean future biomass projections under SSP5-8.5 for (A) arctic (n = 3 species, grey is study area), B arctic–boreal (n = 8), C boreal (n = 40), and D temperate and subtropical (n = 5) species. Projections of SSP1-2.6 and SSP2-4.5 are show in supplementary Figs. 4 and 5. Log CPUE corresponds to log of catch in the survey per unit of effort (Log fish/min).
Among the 7 studied traits, species zoogeography was the best variable in explaining species rate of change of range, core range, and overall biomass. Arctic and boreal-arctic species were projected to decline in the future, and boreal, temperate, subtropical, and deep-water species, to increase (Fig. 6) (multiple linear regression, p < 0.05). Apart from species zoogeography, species trophic level showed a positive effect in species’ range extent, maximum length showed a positive effect in species’ biomass, and maximum depth showed a positive effect in species’ core range (multiple linear regression, p < 0.05).
Projected effect of species zoogeography on annual rate of change from 2010 to 2100 in species’ (A) geographic range, B biomass, C core range, and D core biomass at each socioeconomic pathway. Boxplot black line shows the median change, while box limits correspond to the interquantile range (IQR). Whiskers correspond to maximum and minimum values, calculated as Q3 + 1.5xIQR and Q1 – 1.5xIQR, and points correspond to outliers. Asterisks represent clear effects of each zoogeographic class at a particular shared socioeconomic pathway (multiple linear regression, p < 0.05, see Source Data for individual test results). Biomass units are in catch in the survey per unit effort (CPUE fish/min).
Community-wide range shifts northwards and eastwards were projected across species between 2010 and 2100 (Supplementary Data 2 and 3). Range shifts increased with socioeconomic pathways from a mean of 0.9 km yr−1 northwards and 0.3 km yr−1 eastwards under SSP1-1.26, to 3.2 km yr−1 and 1.1 km yr−1 northwards and eastwards, respectively, under SSP5-8.5 (Supplementary Fig. 6A). Smaller shifts were projected for biomass-weighted centroid shifts, from a mean of 1.0 and 0.8 km yr−1 northwards and eastwards under SSP1-1.26, respectively, to 3.7 km yr−1, and 2.1 km yr−1, respectively, under SSP5-8.5 (Supplementary Fig. 6B). The highest shifts were detected in species’ core range, containing the top 10% of species biomass, for which projected shifts were 1.1 and 0.5 km yr−1 northwards and eastwards, respectively, under SSP1-1.26, to 4.8 km yr−1 and 3.2 km yr−1, respectively, under SSP5-8.5 (Supplementary Fig. 6C).
Geographic range fragmentation
Our results do not show clear trends in habitat fragmentation across species. Although we projected increasing number of polygons per species with increasing socioeconomic pathway, and declines in polygon area (Fig. 7, Supplementary Data 4), these trends are driven by (1) Arctic species declining in polygon area, and (2) warmer-water species increasing in number of polygons (Supplementary Fig. 7). The combination of these two parameters in the same species would lead to increased habitat fragmentation, but each of these processes in different zoogeographic groups suggest no clear trends in habitat fragmentation.
Histograms correspond to species’ (A) rate of change in mean geographic range unit area. B Rate of change in mean area between geographic range units, and C rate of change in distance between geographic range units. Geographic range untis are the individual polygons that compose a species’ projected range. Asterisks indicate significant median different from 0 (Two sided Wilcoxon rank test, p < 0.05, see Source Data for individual test results).
Discussion
We project a drastic reduction of Arctic and boreal-Arctic marine fishes’ ranges and biomasses in the study area with increasingly pessimistic greenhouse gas emission pathways. This may result in the local extirpation of several of those species by within the next decades. Although the expansion of several boreal and warmer-water species leads to an increase in species richness, the present-day projected biomass of Arctic species is not fully replaced by expanding species, resulting in biomass declines across future scenarios. Global ensemble mechanistic modelling efforts conducted in recent years predict increases in consumers biomass in the high Arctic across several taxa, but taxonomic resolution remains a barrier to further interpretation and uncertainty is very high35. Projected increases in biomass, however, could accumulate in different components of the community, and do not necessarily conflict with our projections of overall fish biomass reductions. Moreover, we did not include the effect of fishing impacts in our study, which can act synergistically with climate change and therefore is an important element that needs to be considered in future research, when future predictions of fleet behaviour are developed35.
Polar species are at higher risk of extinction than species from lower latitudes34, and in the case of Arctic demersal fishes, the lack of continental shelf further north than the Barents Sea could represent an additional pressure that limits available habitat at northern latitudes33,36. Thus, the local extirpation of Arctic and Boreal-Arctic demersal species from the Barents Sea could place those species at high risk of global extinction. Among species showing decreasing trends, we project a total extirpation of one boreal-arctic species, the Atlantic poacher (Leptagonus decagonus), under the high emissions scenario. Two out of three Arctic species, the bigeye sculpin (Triglops nybelini) and the pale eelpout (Lycodes pallidus), are projected to suffer local extirpations in the study area not only under the high emissions scenario, but also under the intermediate scenario. The third Arctic species with modelled biomass, polar cod (Boreogadus saida), is projected to lose its relative dominance in all its occurrence area, to the practical disappearance from the study area by the end of the century under the high emissions scenario (residual biomass projected), though strong declines of over 50% in area and biomass are projected under all scenarios. The pelagic nature of polar cod may allow adult individuals to shift to other Arctic regions, some of which may become more suitable for the species in the future37, but its early life stages are highly linked to sea-ice cover, and its recruitment is predicted to collapse with further sea-ice cover reduction38. Whether these and other Arctic or Arctic-boreal species will be able to find refugia by moving to other regions where warming is happening at a slightly slower rate remains to be investigated. This high sensitivity of Arctic and boreal-Arctic fish species to climate warming contrasts with the surprising robustness predicted for Arctic benthic taxa39, which could lead to novel species interactions with overlapping predicted ranges40. However, whether higher trophic levels will be able to shift to other food-sources if Arctic keystone species such as polar cod disappear from the Barents Sea is still unknown (i.e., ringed seals (Phoca hispida) are highly dependent on polar cod41,42).
Overall, our results are in line with the reported ongoing global redistribution of marine species, with increasing richness at higher latitudes1. Our projections suggest that these geographic shifts will lead to biomass increases of several boreal and warmer-water species, and declines of Arctic and Boreal-Arctic species without marked changes in species range fragmentation. These shifts will further contribute to the ongoing borealization of the fish community, and the positive richness trend in the area30,43. We predict that this increase in richness will be accompanied by a shift in the identity of the dominant species, indicating a change in the community which is expected but not always the case44,45, and a general decline in relative dominance. This could have implications in future modelling studies of those communities, as increases in species richness and abundance improve the predictability of community properties45. Moreover, the number of dominant species across the study area declined with time, from 10 species in present-day conditions to 6 species under the high emissions scenario, which points towards a homogenization of the community by the end of the century. Although we project slight declines in richness at lower latitudes of the study area, the interpretation of richness and relative dominance changes in southern regions of the study areas require caution, considering that species expanding their range from outside the study area into the study area are to be expected, but are here not accounted for. Overall, we project future communities with a more balanced share of biomass, higher richness, and lower spatial variation in the dominant species if climate warming exceeds 1.5 °C.
Our results also show that current predictability of Arctic species is particularly challenging due to little data available for model calibration for several Arctic species, suggesting that borrowing information from phylogenetically close species is not enough to obtain informative predictions for those species. Furthermore, SDMs often perform substantially better predicting species static patterns, than predicting changes46. To properly validate the models for predicting species range shifts, their predictive performance should not be tested on their ability to predict static distributions, but on their ability to predict distributional changes46. However, this requires temporally independent data at high-enough spatial resolution to detect distributional changes, and this is missing for most of the species included in this study. Therefore, we believe that there is an urgent need for biomass data collection of shifting species at fine spatial resolution, particularly at species range edges and particularly focusing on Arctic demersal species, which are likely at the front of species risk of global extinction.
The projected increase in biomass of several warmer-water species, linked to the expansion of species from lower latitudes, may represent novel fishing opportunities. For example, ling (Molva molva), monkfish (Lophius budegassa & Lophius piscatorius), whiting (Merlangius merlangus) or haddock (Melanogrammus aeglefinus) are here projected to increase in range and biomass, while mackerel (Scomber scomnrus), a species whose expansion northwards has already led to transboundary conflicts in the northeast Atlantic47, is projected to increase in range at northern latitudes. Interestingly, our projections suggest that cod (Gadus morhua), currently an expanding species in the Barents Sea16, will slightly decline in biomass by the end of the century. This is in line with similar predictions in the North Sea, where although several species of fisheries interest were projected to expand their suitable habitat, cod showed a reduction in habitat suitability by mid-century18. This suggests that for some boreal species, future warming may be of enough magnitude to revert their current expansion trends.
Previous studies in the North Sea, have also identified bottom temperature as the main environmental variable shaping marine fish communities48,49, as have other studies elsewhere50,51,52. Similarly, we identified depth and bottom temperature as the most relevant predictive variables in our study, although the relevance of phytoplankton and dissolved oxygen could have been hindered by the lower resolution of these two variables, which were obtained from a different Copernicus dataset than the rest. Moreover, studies in the North Sea have widely reported climate-warming induced species northward shifts8,29, which resulted in local species richness’ increases53, although some species of commercial interest (e.g., Atlantic cod) may decline in the mid term18,54. Our projections partly point in this direction and show regional slight increases in richness in the North Sea, though also some regional declines. However, special caution is required when interpreting projections in the North Sea for three reasons. First, because future climate in the region has no analogue anywhere in the model calibration and future North Sea conditions are not represented in the study area at present-day conditions, this fraction of the environmental space is poorly sampled during the model calibration. Projections for the less sampled parts of the environmental space are considered less reliable and should be interpreted with greater caution55,56. Second, the historical run of the global earth system model used to obtain environmental data for future projections shows discrepancies with the fitting of environmental data in the North Sea, as the correlation analysis in the supplementary material shows. Finally, in considering richness and dominance estimates, it is highly likely that species expanding their range from outside the study area into the study area concentrate in the North Sea, which is our lowermost region, and these would not be accounted for here. For these reasons, we advise caution in interpreting our results in the southernmost region of this study.
Facing the challenges posed by climate warming, future fishery management strategies must consider shifts in species biomass dynamics and distributions. However, current day modelling techniques and data collection need to be improved for many species to be able to achieve this crucial objective. For example, better modelling of species biomass in our area could help to prevent fisheries conflicts among several economic zones, due to transboundary stock shifts under climate warming9,57,58. Furthermore, fisheries will also affect future marine fish communities, as they have affected marine fish communities in the North and Barents Seas for decades43,59, and current management decisions will contribute to shape fisheries resources in the future, which future modelling approaches will need to include. As such, embracing adaptive management strategies that account for the evolving dynamics of marine ecosystems and fisheries resources is imperative to ensure the sustainability and resilience of our oceans for generations to come.
Methods
Study area and fish biomass data
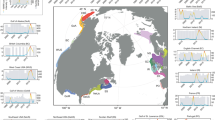
Fish biomass, as relative abundance (catch per unit effort CPUE) was obtained from bottom trawling data collated within the FishGlob database of fish records and biomass standardised with sampling effort, from several international trawl surveys60 (Fig. 1). The data was filtered to the study area (North Sea to Barents Sea), and to the period 2004–2022. This included data from three surveys: the NS-IBTS in the North Sea, the Norwegian Sea coastal survey in the Norwegian Sea and the Nor-BTS in the Barents Sea60. We restricted the analysis to Campelen and GOV trawls, the two main gears used in the North Sea (GOV) and in the Barents Sea (campelen trawling), all gears were equipped with 20 mm mesh size nets bottom trawls, and each haul catch was standardised by effort60.
We restricted the data to the continental shelf, by eliminating the few hauls that were sampled deeper than 500 m (<1% of the hauls), and we eliminated very rare species that were sampled in less than 100 hauls, or in less than 10 years. Finally, some trawls (<1%) were eliminated due to lack of environmental data. The final database included 16,345 unique hauls and included 107 fish species (Supplementary Data 5).
Environmental variables
We selected those physical and biological variables that, based on previous knowledge and expert opinion, are thought to drive the spatial-temporal variability in demersal fish species biomass (Husson et al., 2020). We included (1) surface and (2) bottom water temperature, (3) sea ice concentration for species occurring in areas with sea ice (i.e., not included for strictly temperate species whose model showed very poor convergence for sea ice parameters because no overlap between occurrence/biomass and sea ice, (Supplementary data 5), (4,5) currents (northward and eastward components), (6) bottom dissolved oxygen concentration, (7) phytoplankton concentration, and (8) water depth.
Sea ice concentration, surface and bottom temperatures, and northward and eastward current components were obtained from the Global Ocean Physics Reanalysis at a resolution of 0.08° × 0.08°, while bottom dissolved oxygen, and bottom primary productivity were obtained from the Global Ocean Biogeochemistry Hindcast at a resolution of 0.25° × 0.25°, both of which were available through the Marine Copernicus repository61,62. Bottom depth was obtained from BioOracle at a resolution of 0.08°63. Environmental information was extracted for each sampling point corresponding to the monthly mean of each survey month, except for sea ice, where the annual mean was preferred, because winter sea ice dynamics can highly influence the populations of several Barents Sea marine fishes throughout the year (Supplementary Table 1).
To remove co-linear variables, which can increase uncertainty and decrease statistical power of the models64, we calculated the Variance Inflated Factor (VIF), and eliminated all variables using a conservative threshold VIF greater than 465. This led us to eliminate surface temperature, as it was highly correlated with bottom temperature, leaving 7 environmental variables to include in the model. Moreover, for bottom temperature and depth, we included their second-order polynomial responses, to allow a bell-shape response of species distribution and biomass to these variables66.
Future environmental layers
Future mean annual environmental layers were obtained from the second version of the Institute Pierre Simon Laplace climate model (IPSL- CMIP6)67, which is the only model within the Coupled Model Intercomparison Project Phase 6 (CMIP6) that contained all our predictive variables. We used future environmental data from three different shared socioeconomic pathways (SSPs): the most optimistic ‘high mitigation’ scenario reflecting sustainable development and social justice, where the probability of exceeding +2 °C by 2100 is kept below 33% (SSP1-2.6, + 1.6 °C by 2100), an ‘intermediate scenario’ (SSP2-4.5, + 2.6 °C by 2100), and a scenario reflecting a world of rapid growth and without restrictions on economic production and the use of energy, the ‘high emissions’ scenario (SSP5-8.5, + 4.5 °C by 2100)68.
Mean annual environmental data layers for each scenario (SSP1-2.6, SSP2-4.5 and SSP5-8.5) were extracted in 10-year increments and used for predicting species’ distributions. For comparison with the present-day conditions, we used the mean between 2010:2013 to represent present-day conditions, leading to an overall of 25 time periods (8 future periods × 3 scenarios, and 1 present-day scenario).
Statistical modelling
A two-part hurdle model was used to model the distribution of demersal fish biomass dealing with the excess number zeroes in the dataset69. In this procedure, a binomial model with a probit distribution was used to project the probability of species’ occurrence, and a separate model with a Gaussian distribution was fit to species’ log transformed catch per unit effort, to project species’ biomass. This was done using the Hierarchical Modelling of Species Communities JSDM approach (in the ‘Hmsc’ package in R, Tikhonov et al.70).
In both models (binomial and gaussian), spatial autocorrelation was accounted for by including a spatially explicit random effect using Gaussian Predictive Processes (GPP assuming that information on the spatial structure of the data can be summarized at a smaller number of (in our case 183) ‘knot’ locations71. The inclusion of spatial autocorrelation when fitting the model is highly recommended to improve the estimation of the model coefficients72.
Although we excluded the rarest species from the analysis, most of the species remaining can still be considered rare (65% were present in <10% of the hauls). This poses a challenge in estimating the realized niche of those species, which only have few points for estimating the limits of their environmental niche. However, species inhabit environments that share some similarities with those of their close relatives, because they follow niche conservatism to some degree73. In the HMSC framework, the statistical relationships between species occurrences or biomass with the environment are integrated through a hierarchical structure that allowed us to determine to what extent environmental filtering is structured by species phylogenetic relationship, and species traits70. This is done by including a phylogenetic correlation parameter rho that measures if the residual variation of species responses to the environment is phylogenetically structured. For this reason, we built a basic taxonomic tree using the NCBI Common Tree software, available through its website74, which is the best available proxy for phylogenetic relatedness when species phylogenetic data are not available with very similar relationships to formal taxonomic classifications. Strong phylogenetic signals may point to response traits that have not been specifically accounted for in the model.
We analysed projected species distributions to study whether some species sharing traits responded similarly in range expansion and/or contraction using backward selection multiple regression analysis. We selected eight species’ biological traits that could be related to species expansion potential29,75 including five functional traits: (1) maximum length (cm), (2) age at maturity (years), (3) fecundity (number of eggs), (4) habitat (demersal or pelagic), and (5) trophic level; one physiological trait (6) preferred temperature (°C); and one bathymetric trait (7) maximum depth. Traits for each demersal fish species were obtained from FishBase76. We finally created a zoogeography trait (8) assigning a general climatic classification for each species, of the following categories: ‘Arctic’, ‘Arctic-Boreal’, ‘Boreal’, or ‘Deepwater’, as classified in Mecklenburg et al.77, or from FishBase when the species were not present in the former reference (i.e., not present in Arctic latitudes), adding the categories ‘subtropical’ and ‘temperate’ to the list of possible categories76.
Model fitting was conducted using the Markov Chain Monte Carlo (MCMC) implemented in Hmsc. Model convergence was assessed using the Gelman-Rubin Potential Scale Reduction Factor78. Four MCMC chains were run, each collecting 250 samples, applying a thinning of 500, and the first 62 500 runs were discarded as burn in. The MCMC convergence was satisfactory, as indicated by a mean scale reduction factor of all parameters <1.1, and the effective sample size of the MCMC was close to the number of posterior samples, indicating no major issues of sample autocorrelation. Model goodness of fit was then assessed by computing the overall explanatory capability (calculated from the species’ data used in the HMSC model fitting) as the mean AUC and r2 value across all species-specific values. To evaluate the predictive performance of the model, a five-fold cross-validation was undertaken (i.e., assessing the HMSC model fit using the withheld data from each fold). The model fitting, calibration and validation was done using the ‘Hmsc’ package in R70, and code from66,79.
The explanatory power of the model had a mean AUC of 0.97 for the presence-absence model, and an R2 of 0.54 for the biomass model, while the mean predictive power from a five-fold cross validation was lower (AUC of 0.88, and a mean R2 of 0.12) (Supplementary data 5). Species richness projected using present-day layers was significantly correlated with surveyed species richness (Pearson r = 0.4, p < 0.01).
To show the discrepancy between the fitting and the predictive datasets (fitting was done with Copernicus datasets, and projections with IPSL global earth model), we conducted a correlation analysis between both sources of SBT monthly averages, in the overlapping period of the IPSL historical run, and the SBT data from Copernicus for the coordinates included in this study. This includes all our data between 2004 and 2014. We show that both datasets are significantly correlated, but present discrepancies in the North Sea (Person correlation = 0.61 Pearson correlation excluding North Sea = 0.85) (Supplementary Fig. 8). Moreover, we conducted a multivariate environmental similarity surface (MESS) analysis using the MESS() function from the modEvA package in R80, to assess whether the ‘environmental space’ in our projections was accordingly sampled during the model training (Supplementary Fig. 9). The ‘environmental space’ is the multidimensional space produced by considering each of the environmental variables as a dimension. Projections in poorly sampled parts of the environmental space are considered less reliable (strongly negative MESS values), and should be interpreted with greater caution56,81.
Finally, we examined the patterns of species co-occurrences at the level of the spatial random effect. The co-occurrence of specific species is drawn from the covariance structure of the model residuals once the fixed environmental effects have been considered. This analysis reveals pairs of species that either co-occur more frequently or less frequently than expected by random chance, which can partly represent biotic interactions25,82.
Biomass models: From the initial 107 species included in the model, we restricted all biomass-based analyses to species with > 0.05 mean R2 in the five-fold cross validation biomass model.
Although explaining only 5% of the variance of the data may seem a rather poor fit, two things need to be considered: First, the distribution of the biomass is determined by the occurrence model which shows substantially better predictive performance. Second, the threshold of 5% is arbitrary, but not random. All models with a CV R2 higher than 0% are informative, but because every fold of the CV provides a different value, and most of the average values close to 0% have some folds with negative percentages, we chose a more conservative threshold of 5%. This resulted in the inclusion of 61 species with mean R2 of 0.22, ranging from 0.05 to 0.53 (Supplementary data 5). We multiplied the projections from the gaussian log biomass model with the projections from the binomial model to obtain the final biomass projections. Then, we assigned 0 to all projected biomass lower than the minimum value recorded in the dataset, divided by 2. This was done to assign a threshold for presence-absence of species that is biologically meaningful (minimum recorded) while assuming certain presence below detectability (divided by 2).
Occurrence models: All 107 species included in our study had reasonable predictive performance of presence-absence models (lowest AUC = 0.67 in five-fold cross validation). For this reason, we included all species in those analysis that required only presence-absence information (i.e., changes in species geographic range, and species richness). To threshold presence-absence in those analyses, we calculated the threshold of probability of occurrence that maximised the True Skill Statistic per species83, and we used that species-specific threshold to assign presence or absence of each species across the study area.
Spatial projections: We projected geographic distributions of species’ occurrence and biomass across the period 2010:2013 (historic reference, which we refer to as ‘present-day’ projections) and from 2030 to 2100 every 10th year for three different possible climate change scenarios (SSP1-2.6, SSP2-4.5 and SSP5-8.5). Although our model included spatial processes for estimating its parameters (using the GPP methodology explained above), future projections excluded the spatial random effect. This was done consciously, to avoid extrapolating estimated spatial structures that may not persist under future climatic conditions.
Geographic range metrics
Four species geographic distribution indicators were used to explore changes in distribution over time for each climate change scenario. First, the ‘range’ of a species was measured as the area (km2) of projected occurrence (based on the thresholded spatial projections from the presence-absence model). The second measure ‘biomass’ quantified the total projected species’ log CPUE across the study area (based on the thresholded spatial projections from the hurdle model). Third, the ‘core range’ represented areas with the highest biomass. Core range was identified using the 90th quantile of present-day species’ biomass (that is we selected cells that contain the top 10% of CPUE). Future core range was estimated by selecting all areas where future CPUE was ≥ than the present-day 90th quantile CPUE value. Finally, the fourth measure ‘core biomass’ refers to the total biomass (CPUE) within the core range. These measures were calculated by projecting the model into the equal-area Lambert azimuthal projection, and we studied their rate of change regressing them with time (using linear regression). The analysis of the data was conducted using the ‘raster’ and ‘rgdal’ packages in R, and plotting was done using tidyverse and ggspatial, while the MESS analysis was conducted using the modEvA package80,84,85,86,87,88.
To study potential changes in geographic range fragmentation, we converted each species’ projected range to polygons, and calculated the number of polygons, the mean area, and the mean distance between polygons of each species (Supplementary Data 4). Many small polygons with large mean distance between them would represent a very fragmented range, while few, big polygons, close to each other, would represent a less fragmented range.
Species richness and relative dominance
Species richness was calculated by summing all projected probabilities of occurrences across species (n = 107)89. Dominance was calculated as the percent contribution of the highest species’ CPUE to the total CPUE (sum of all CPUE of all species) at each cell, and dominant species are the species with the highest biomass in each cell.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data used in this study was obtained from bottom trawling data collated within the FishGlob60 (Accessible at https://github.com/AquaAuma/fishglob_data). The Norwegian Sea section of this data is no longer available in FishGlob, and needs to be directly asked for to the Norwegian Marine Data Centre (https://metadata.nmdc.no/metadata-api/landingpage/f77112db062b5924d079a54b311260fb). The environmental data used for calibrating the model came from the ‘Global Ocean Physics Reanalysis’ and the ‘Global Ocean Biogeochemistry Hindcast’ both of which were available through the Marine Copernicus repository61,62 at https://data.marine.copernicus.eu/products. Bottom depth was obtained from BioOracle63 at https://www.bio-oracle.org. The environmental data used for future projections came from the second version of the IPSL climate model (IPSL- CMIP6)67, and is fully accessible as well at https://esgf-data.dkrz.de/search/cmip6-dkrz/. World administrative boundaries polygons are available from opendatasoft, accessible at: https://public.opendatasoft.com/explore/dataset/world-administrative-boundaries/information90. The data generated in this study, and used for Figs. 3, 6 and 7 is provided in the Source Data file, while the output of all regression analysis are available in the Supplementary Data files, as well as the individual species range and biomass projections. The trait database gathered is available at https://github.com/CescGV/JSDM-Barents-Norwegian-North91. Source data are provided with this paper.
Code availability
Code used for this publication is available at https://github.com/CescGV/JSDM-Barents-Norwegian-North91.
References
Chaudhary, C., Richardson, A. J., Schoeman, D. S. & Costello, M. J. Global warming is causing a more pronounced dip in marine species richness around the equator. Proc. Natl. Acad. Sci. USA 118, e2015094118 (2021).
Poloczanska, E. S. et al. Global imprint of climate change on marine life. Nat. Clim. Chang. 3, 919–925 (2013).
Sunday, J. M., Bates, A. E. & Dulvy, N. K. Thermal tolerance and the global redistribution of animals. Nat. Clim. Chang. 2, 686–690 (2012).
Pinsky, M. L., Worm, B., Fogarty, M. J., Sarmiento, J. L., & Levin, S. A. Marine taxa track local climate velocities. Science. 341, 1239–1242 (2013).
Rantanen, M. et al. The Arctic has warmed nearly four times faster than the globe since 1979. Commun. Earth Environ. 3, 1–10 (2022).
Batt, R. D., Morley, J. W., Selden, R. L., Tingley, M. W. & Pinsky, M. L. Gradual changes in range size accompany long-term trends in species richness. Ecol. Lett. 20, 1148–1157 (2017). at.
Cheung, W. W. L. et al. Projecting global marine biodiversity impacts under climate change scenarios. Fish Fish 10, 235–251 (2009).
Gordó-Vilaseca, C. et al. Over 20% of marine fishes shifting in the North and Barents Seas, but not in the Norwegian Sea. PeerJ 11, 1–23 (2023).
Engelhard, G. H., Righton, D. A. & Pinnegar, J. K. Climate change and fishing: a century of shifting distribution in North Sea cod. Glob. Chang. Biol. 20, 2473–2483 (2014).
Payne, M. R., Kudahl, M., Engelhard, G. H., Peck, M. A. & Pinnegar, J. K. Climate risk to European fisheries and coastal communities. Proc. Natl Acad. Sci. USA 118, 1–10 (2021).
Lavin, C. P., Gordó-Vilaseca, C. & Costello, M. J. Global fisheries in a warming world. in Reference Module in Earth Systems and Environmental Sciences (Elsevier, 2021). https://doi.org/10.1016/b978-0−12-821139-7.00096-9.
Cheung, W. W. L., Reygondeau, G. & Frölicher, T. L. Large benefits to marine fisheries of meeting the 1.5 °C global warming target. Science 354, 1591–1594 (2016).
Pinsky, M. L. et al. Preparing ocean governance for species on the move. Science 360, 1189–1191 (2018).
Ramírez, F., Shannon, L. J., Angelini, R., Steenbeek, J. & Coll, M. Overfishing species on the move may burden seafood provision in the low-latitude Atlantic Ocean. Sci. Total Environ. 836, 155480 (2022).
Cormon, X., Kempf, A., Vermard, Y., Vinther, M. & Marchal, P. Emergence of a new predator in the North Sea: evaluation of potential trophic impacts focused on hake, saithe, and Norway pout. ICES J. Mar. Sci. 73, 1370–1381 (2016).
Kortsch, S., Primicerio, R., Fossheim, M., Dolgov, A. V. & Aschan, M. Climate change alters the structure of arctic marine food webs due to poleward shifts of boreal generalists. Proc. R. Soc. B Biol. Sci. 282, 20151546 (2015).
Schmidt, D. N., Pieraccini, M. & Evans, L. Marine protected areas in the context of climate change: key challenges for coastal social-ecological systems. Philos. Trans. R. Soc. B: Biol. Sci. 377, 20210131 (2022).
Townhill, B. L., Couce, E., Tinker, J., Kay, S. & Pinnegar, J. K. Climate change projections of commercial fish distribution and suitable habitat around north western Europe. Fish Fish 24, 848–862 (2023).
Katara, I. et al. Conservation hotspots for fish habitats: a case study from English and Welsh waters. Reg. Stud. Mar. Sci. 44, 101745 (2021).
Payne, M. R. et al. Skilful decadal-scale prediction of fish habitat and distribution shifts. Nat. Commun. 13, 2660 (2022).
D’Amen, M., Rahbek, C., Zimmermann, N. E. & Guisan, A. Spatial predictions at the community level: from current approaches to future frameworks. Biol. Rev. 92, 169–187 (2015).
Ovaskainen, O. et al. How to make more out of community data? A conceptual framework and its implementation as models and software. Ecol. Lett. 20, 561–576 (2017).
Pichler, M. & Hartig, F. A new joint species distribution model for faster and more accurate inference of species associations from big community data. Methods Ecol. Evol. 12, 2159–2173 (2021).
Zurell, D., Pollock, L. J. & Thuiller, W. Do joint species distribution models reliably detect interspecific interactions from co-occurrence data in homogenous environments? Ecography 41, 1812–1819 (2018).
Norberg, A. et al. A comprehensive evaluation of predictive performance of 33 species distribution models at species and community levels. Ecol. Monogr. 89, e01370 (2019).
Ingvaldsen, R. B. et al. Physical manifestations and ecological implications of Arctic Atlantification. Nat. Rev. Earth Environ. 2, 874–889 (2021).
Weinert, M. et al. Modelling climate change effects on benthos: distributional shifts in the North Sea from 2001 to 2099. Estuar. Coast. Shelf Sci. 175, 157–168 (2016).
Montero-Serra, I., Edwards, M. & Genner, M. J. Warming shelf seas drive the subtropicalization of European pelagic fish communities. Glob. Chang. Biol. 21, 144–153 (2015).
Perry, A. L., Low, P. J., Ellis, J. R. & Reynolds, J. D. Ecology: Climate change and distribution shifts in marine fishes. Science. 308, 1912–1915 (2005).
Gordó-Vilaseca, C., Stephenson, F., Coll, M., Lavin, C. & Costello, M. J. Three decades of increasing fish biodiversity across the northeast Atlantic and the Arctic Ocean. Proc. Natl. Acad. Sci. USA 120, e2120869120 (2023).
Kortsch, S. et al. Climate-driven regime shifts in Arctic marine benthos. Proc. Natl. Acad. Sci. USA 109, 14052–14057 (2012).
Frainer, A. et al. Climate-driven changes in functional biogeography of Arctic marine fish communities. Proc. Natl Acad. Sci. USA 114, 12202–12207 (2017).
Kitchel, Z. J., Conrad, H. M., Selden, R. L. & Pinsky, M. L. The role of continental shelf bathymetry in shaping marine range shifts in the face of climate change. Glob. Chang. Biol. 28, 5185–5199 (2022).
Penn, J. L. & Deutsch, C. Avoiding ocean mass extinction from climate warming. Science. 376, 524–526 (2022).
Tittensor, D. P. et al. Next-generation ensemble projections reveal higher climate risks for marine ecosystems. Nat. Clim. Chang. 11, 973–981 (2021).
Rutterford, L. A. et al. Future fish distributions constrained by depth in warming seas. Nat. Clim. Chang. 5, 569–573 (2015).
Geoffroy, M. et al. The circumpolar impacts of climate change and anthropogenic stressors on Arctic cod (Boreogadus saida) and its ecosystem. Elementa 11, 1–44 (2023).
Huserbråten, M. B. O., Eriksen, E., Gjøsæter, H. & Vikebø, F. Polar cod in jeopardy under the retreating Arctic sea ice. Commun. Biol. 2, 1–8 (2019).
Renaud, P. E. et al. Arctic sensitivity? Suitable habitat for benthic taxa is surprisingly robust to climate change. Front. Mar. Sci. 6, 538 (2019).
Pecuchet, L. et al. Novel feeding interactions amplify the impact of species redistribution on an Arctic food web. Glob. Chang. Biol. 26, 4894–4906 (2020).
Hop, H. & Gjøsæter, H. Polar cod (Boreogadus saida) and capelin (Mallotus villosus) as key species in marine food webs of the Arctic and the Barents Sea. Mar. Biol. Res. 9, 878–894 (2013).
Sakshaug, E., Bjørge, A., Gulliksen, B., Loeng, H. & Mehlum, F. Structure, biomass distribution, and energetics of the pelagic ecosystem in the Barents Sea: A synopsis. Polar Biol. 14, 405–411 (1994).
Johannesen, E. et al. Changes in Barents Sea ecosystem state,1970–2009:climate fluctuations,human impact, and trophic interactions. ICES J. Mar. Sci. 69, 880–889 (2012).
Costello, M. & Myers, A. Amphipod fauna of the sponges Halichondria panicea and Hymeniaci-don perleve in Lough Hyne. Irel. Mar. Ecol. Prog. Ser. 41, 115–121 (1987).
Dornelas, M., Phillip, D. A. T. & Magurran, A. E. Abundance and dominance become less predictable as species richness decreases. Glob. Ecol. Biogeogr. 20, 832–841 (2011).
Piirainen, S. et al. Species distributions models may predict accurately future distributions but poorly how distributions change: a critical perspective on model validation. Divers. Distrib. 29, 654–665 (2023).
Spijkers, J. & Boonstra, W. J. Environmental change and social conflict: the northeast Atlantic mackerel dispute. Reg. Environ. Chang. 17, 1835–1851 (2017).
Montanyès, M., Weigel, B. & Lindegren, M. Community assembly processes and drivers shaping marine fish community structure in the North Sea. Ecography. 2023, 1–14 (2023).
Rutterford, L. A., Simpson, S. D., Bogstad, B., Devine, J. A. & Genner, M. J. Sea temperature is the primary driver of recent and predicted fish community structure across Northeast Atlantic shelf seas. Glob. Chang. Biol. 29, 2510–2521 (2023).
Pecuchet, L. et al. Spatio-temporal turnover and drivers of bentho-demersal community and food web structure in a high-latitude marine ecosystem. Divers. Distrib. 28, 2503–2520 (2022).
Punzón, A. et al. Tracking the effect of temperature in marine demersal fish communities. Ecol. Indic. 121, 107142 (2021).
Maynou, F., Sabatés, A. & Salat, J. Clues from the recent past to assess recruitment of Mediterranean small pelagic fishes under sea warming scenarios. Clim. Change 126, 175–188 (2014).
Jones, D. I., Miethe, T., Clarke, E. D. & Marshall, C. T. Disentangling the effects of fishing and temperature to explain increasing fish species richness in the North Sea. Biodivers. Conserv. 32, 3133–3155 (2023).
Chaikin, S., Riva, F., Marshall, K. E., Lessard, J. P. & Belmaker, J. Marine fishes experiencing high-velocity range shifts may not be climate change winners. Nat. Ecol. Evol. https://doi.org/10.1038/s41559-024-02350-7 (2024).
Stephenson, F. et al. Modelling the spatial distribution of cetaceans in New Zealand waters. Divers. Distrib. 26, 495–516 (2020).
Owens, H. L. et al. Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecol. Modell. 263, 10–18 (2013).
Mendenhall, E. et al. Climate change increases the risk of fisheries conflict. Mar. Policy 117, 103954 (2020).
Haug, T. et al. Future harvest of living resources in the Arctic Ocean north of the Nordic and Barents Seas: a review of possibilities and constraints. Fish. Res. 188, 38–57 (2017).
Jennings, S., Blanchard, J. L., Jennings, S. & Blanchard, J. L. Fish abundance with no fishing: predictions based on macroecological theory. J. Animal Ecol. 73, 632–642 (2004).
Maureaud, A. A. et al. FishGlob_data: An Integrated Database of Fish Biodiversity Sampled With Scientific Bottom-trawl Surveys. (National Marine Fisheries Service (NOAA), 2023). https://osf.io/2bcjw/.
European Union-Copernicus Marine Service. Global Ocean Physics Reanalysis [Data set]. (Mercator Océan International, 2023). https://doi.org/10.48670/MOI-00021.
European Union-Copernicus Marine Service. Global Ocean Biogeochemistry Hindcast [Data set]. (Mercator Océan International, 2023). https://doi.org/10.48670/moi-00019.
Assis, J. et al. Bio-ORACLE v2.0: Extending marine data layers for bioclimatic modelling. Glob. Ecol. Biogeogr. 27, 277–284 (2018).
De Marco, P. & Nóbrega, C. C. Evaluating collinearity effects on species distribution models: An approach based on virtual species simulation. PLoS One 13, e0202403 (2018).
O’Brien, R. M. A caution regarding rules of thumb for variance inflation factors. Qual. Quant. 41, 673–690 (2007).
Ovaskainen, O. & Abrego, N. Joint Species Distribution Modelling: With Applications in R. (Cambridge University Press, 2020). https://doi.org/10.1017/9781108591720.
Boucher, O. et al. IPSL IPSL-CM6A-LR model output prepared for CMIP6 CMIP. https://doi.org/10.22033/ESGF/CMIP6.1534 (2018).
Riahi, K. et al. The Shared Socioeconomic Pathways and their energy, land use, and greenhouse gas emissions implications: an overview. Glob. Environ. Chang. 42, 153–168 (2017).
Welsh, A. H., Cunningham, R. B., Donnelly, C. F. & Lindenmayer, D. B. Modelling the abundance of rare species: statistical models for counts with extra zeros. Ecol. Model. 88, 297–308 (1996).
Tikhonov, G. et al. Joint species distribution modelling with the r-package Hmsc. Methods Ecol. Evol. 11, 442–447 (2020).
Tikhonov, G. et al. Computationally efficient joint species distribution modeling of big spatial data. Ecology 101, e02929 (2020).
Dormann, C. F. Effects of incorporating spatial autocorrelation into the analysis of species distribution data. Glob. Ecol. Biogeogr. 16, 129–138 (2007). at.
Wiens, J. J. & Graham, C. H. Niche conservatism: Integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol., Evol. Syst. 36, 519–539 (2005). at.
NCBI. Common Taxonomy Tree. https://www.ncbi.nlm.nih.gov/Taxonomy/CommonTree/wwwcmt.cgi (2017).
Sunday, J. M. et al. Species traits and climate velocity explain geographic range shifts in an ocean-warming hotspot. Ecol. Lett. 18, 944–953 (2015).
Froese, R. & Pauly, D. Fishbase. World Wide Web electronic publication. FishBase (2023).
Mecklenburg, C. W. et al. Marine fishes of the Arctic region. Conservation of Arctic Flora and Fauna II (Conservation of Arctic Flora and Fauna, 2018).
Gelman, A. & Rubin, D. B. Inference from iterative simulation using multiple sequences. Stat. Sci. 7, 457–472 (1992).
Stephenson, F. et al. Using joint species distribution modelling to predict distributions of seafloor taxa and identify vulnerable marine ecosystems in New Zealand waters, PREPRINT (Version 1). Research Square. Available from: https://doi.org/10.21203/rs.3.rs-3457413/v1 (2023)
Barbosa, A. M., Real, R., Munoz, A. R. & Brown, J. A. New measures for assessing model equilibrium and prediction mismatch in species distribution models. Divers. Distrib. 19, 1333–1338 (2013).
Elith, J., Kearney, M. & Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 1, 330–342 (2010).
Poggiato, G. et al. On the interpretations of joint modeling in community ecology. Trends Ecol. Evol. 36, 391–401 (2021).
Allouche, O., Tsoar, A. & Kadmon, R. Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 43, 1223–1232 (2006).
Hijmans, R.J. Raster: Geographic Data Analysis and Modeling. R Package Version 2.4-15. http://CRAN.R-project.org/package=raster (2015)
Bivand, R., Keitt, T. & Rowlingson, B. rgdal: Bindings for the ‘Geospatial’ Data Abstraction Library. (2023).
R Development Core Team. R: A Language and Environment for Statistical Computing. (Foundation for Statistical Computing, Vienna, Austria, 2017). http://www.R-project.org.
Wickham, H. et al. Welcome to the ‘tidyverse’. J. Open Source Softw. 4, 1686 (2019).
Dunnington, D. ggspatial: Spatial Data Framework for ggplot2. https://paleolimbot.github.io/ggspatial/, https://github.com/paleolimbot/ggspatial (2023).
Calabrese, J. M., Certain, G., Kraan, C. & Dormann, C. F. Stacking species distribution models and adjusting bias by linking them to macroecological models. Glob. Ecol. Biogeogr. 23, 99–112 (2014).
UN. World Administrative Boundaries - Countries and Territories. https://public.opendatasoft.com/explore/dataset/world-administrative-boundaries/information/ (2019).
Gordó-Vilaseca, C. Future Trends of Marine Fish Biomass Distributions from the North Sea to the Barents Sea. https://doi.org/10.5281/zenodo.11395792 (2024).
Acknowledgements
C.G.V. would like to thank Lars Martin Jakt from Nord University, for introducing me to bash, tmux and emacs. Thanks to Aleksandra Elbakian for always being supportive and providing us with great input. MC would like to acknowledge partial funding from the Spanish National Project ProOceans (PID2020−118097RB-I00) and institutional support of the ‘Severo Ochoa Centre of Excellence’ accreditation (CEX2019-000928-S).
Funding
Open access funding provided by Nord University.
Author information
Authors and Affiliations
Contributions
C.G.V.: Conceptualization, Visualisation, Data clenaing, model construction and analysis, writing – original draft; M.J.C.: Conceptualization, writing – review & editing; M.C.: Conceptualization, Writing – review & editing; A.J.: Conceptualization, Writing – review & editing; H.R.: Conceptualization, Writing – review & editing; F.S.: Conceptualization, model construction, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gordó-Vilaseca, C., Costello, M.J., Coll, M. et al. Future trends of marine fish biomass distributions from the North Sea to the Barents Sea. Nat Commun 15, 5637 (2024). https://doi.org/10.1038/s41467-024-49911-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-49911-9
- Springer Nature Limited