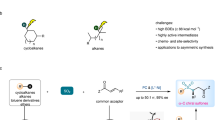
Abstract
The generation of alkyl radical from C(sp3)−H substrates via hydrogen atom abstraction represents a desirable yet underexplored strategy in alkylation reaction since involving common concerns remain adequately unaddressed, such as the harsh reaction conditions, limited substrate scope, and the employment of noble metal- or photo-catalysts and stoichiometric oxidants. Here, we utilize the synergistic strategy of photoredox and hydrogen atom transfer (HAT) catalysis to accomplish a general and practical functionalization of unactived C(sp3)−H centers with broad reaction scope, high functional group compatibility, and operational simplicity. A combination of validation experiments and density functional theory reveals that the N-centered radicals, generated from free N − H bond in a stepwise electron/proton transfer event, are the key intermediates that enable an intramolecular 1,5-HAT or intermolecular HAT process for nucleophilic carbon-centered radicals formation to achieve heteroarylation, alkylation, amination, cyanation, azidation, trifluoromethylthiolation, halogenation and deuteration. The practical value of this protocol is further demonstrated by the gram-scale synthesis and the late-stage functionalization of natural products and drug derivatives.
Similar content being viewed by others
Introduction
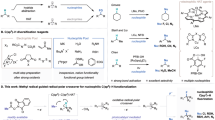
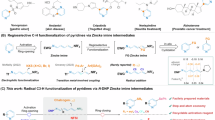
The direct functionalization of unactivated C(sp3)−H bonds has long been a focal point of chemical synthesis, allowing for the rapid construction of C(sp3)−X (X = carbon or heteroatom) bonds in natural products and valuable drugs in a convenient and high atom-economic manner1. However, the intrinsic inertness of aliphatic C−H bonds as well as regioselectivity of multiple C−H bonds of similar chemical environments in feedstock alkanes, has posed formidable challenges for the development of highly demanding catalytic systems2,3,4. The past decades has witnessed tremendous development of inert aliphatic C−H bonds activation with the assistance of transition metals and directing groups, yet a high degree of unmet need remains5,6,7. Eminently, the strategy that synergistically utilizing photoredox and HAT catalysis with electrophilic heteroatom-centered radicals (halogen, N, O, and S) has offered a complementary and potential avenue for the selective activation of inert aliphatic C−H bonds and subsequent functionalization8,9,10, which was proverbially employed in well-established Hofmann-Löffler-Freytag (HLF) reaction11. Notwithstanding remarkable progress in this domain, the reactivity in this transformation is mainly restricted by the inherent difficulties in the formation of the heteroatom-centered radical species with directional effect, such as nitrogen-centered radicals (NCRs), which were generated typically from the prefunctionalized precursors of free N−H bond, such as N−halogen12,13, N−N14, N−O15,16,17, and N−S18,19 precursors (Fig. 1a). In contrast, the generation of NCRs from free amines represents the most straightforward and desirable strategy but is thermodynamically challenging owing to the extraordinary stability of the N−H bonds (BDFEs > 100 kcal/mol)20,21. In this context, oxidative proton-coupled electron transfer (PCET) catalysis has gradually emerged as a reliable strategy for the general activation of N−H bonds22,23,24,25, yet the application in HLF reaction is currently limited to the construction of remote carbon-carbon bonds (Fig. 1b)26,27,28,29,30,31,32. Thus, there still exists a clear impetus for developing a practical and robust photoredox catalytic platform that (1) can directly generate NCRs from non-prefunctionalized N−H bonds and (2) realize the diversified application of subsequent functionalization of unactivated aliphatic C−H bond in a site-selective manner.
a N-directed remote C(sp3)−H activation from N-heteroatom precursors. b Remote C(sp3)−H activation from free amines based on PCET. c Our hypothesis: remote C(sp3)−H activation from free amines based on ET/PT. d Reaction design. e This work: remote C(sp3)−H functionalization through ET/PT. PG protecting group, FG functional group, EWG electron-withdrawing group.
In the relentless exploration of photocatalysis, a mechanistically distinct photocatalytic mode caught our attention, namely sequential electron/proton transfer (ET/PT), which can enable the activation of free N−H bonds to access high-energy NCRs. However, existing research based on this potent platform was mainly focused on the NCRs-engaged coupling or cascade cyclization to construct nitrogen-carbon bonds33,34. Based on the capability of NCRs to serve as the C(sp3)−H bond activators and the enormous potential of photoredox catalysis in the field of synthetic chemistry, we questioned whether an efficient, unactivated, selective C–H functionalization of aliphatic precursors could be achieved through photocatalytic ET/PT mode (Fig. 1c). On the other hand, the Minisci reaction involving the coupling between heteroarenes and nucleophilic alkyl radicals, is a powerful method to achieve the heteroarene diversification simply35. However, owing to the strong aliphatic C−H bonds and the net oxidative nature36,37, previous studies on Minisci-type reaction between heterocycles and C(sp3)−H donors were limited by the employment of prefunctionalized C(sp3)−H substrates, precious catalysts, and excessive amount of oxidant. Encouraged by the recent advancement in hydrogen-evolution cross coupling via the utilization of synergistic catalysis that combines photoredox-capable catalysts and transition metals38,39,40, we believe that merging this synergistic catalysis manifold with the Minisci alkylation would afford an opportunity to overcome the above obstacles.
Herein, we disclose a photoredox-cobalt dual-catalyzed site-selective heteroarylation of unactive C(sp3)−H centers, in which N-centered sulfonamidyl radical intermediates generated through cleavage of N−H bonds in a photoinduced stepwise ET/PT process, are the key HAT catalysts for nucleophilic carbon-centered radical formation. A depiction of our reaction design appears in Fig. 1d. Given the extensive application of acridine-based photocatalysts in the functionalization of C(sp3)−H bonds due to their ability to produce active HAT radical species through photoinduced single electron oxidation41,42,43, we thus envisioned that, in the presence of blue light (450-460 nm), the excited state of 9-mesityl-10-methylacridinium perchlorate (Mes-Acr+ClO4-) (E*p/2 = +2.06 V vs SCE), is of oxidant enough to remove an electron from neutral amine substrate to afford the nitrogen radical cation A, which then further deproton to produce N-centered radical B. A commercially accessible cobaloxime catalyst [Co(dmgH)2Py]Cl was introduced to recover the ground state of Mes-Acr+ from Mes-Acr•. The so-formed nitrogen radical could trigger intramolecular remote HAT through a cyclic transition state to afford the distal alkyl radical C. Subsequently, the addition of alkyl radical C to protonated heteroarenes would give the alkylated intermediate D, which then remove a hydrogen atom in the catalysis of photochemically generated CoII species to deliver the target product E and hydrogen. In addition, the successful development in remote alkylation, amination, cyanation, azidation, trifluoromethylthiolation, halogenation, and deuteration of N-alkylsulfonamides based on ET/PT catalytic platform further emphasizes its versatility in synthetic chemistry (Fig. 1e), which is not only to realize the construction of useful structures but also expected to expedite the expansion of photoinduced ET/PT strategy in remote C(sp3)−H functionalization.
Results
Evaluation of the reaction conditions
To verify the feasibility of the vision, 2-phenylquinoline 1a and 4-methoxy-N-pentylbenzenesulfonamide 2a were chosen as model substrates in the presence of 2.0 equivalents trifluoroacetic acid (TFA) and a catalytic amount of Mes-Acr+ClO4- (PC-1) and cobaloxime [Co(dmgH)2Py]Cl in ACN at room temperature (Table 1). To our delight, the desired remote-coupled product 3 could be obtained in 62% isolated yield by reacting under the irradiation of blue light for 24 h (entry 1). Increasing the loading amount of photocatalyst and cobaloxime catalyst did not significantly improve the productivity of 3 (entries 2–3). Switching PC-1 with PC-2 resulted in slightly lower yields, whereas the excited reduction potential of PC-2 (E*p/2 = +2.20 V vs SCE) was more positive than PC-1 (entry 4). The yield of product 3 was dramatically diminished when employing PC-3, PC-4, or PC-5 as photocatalysts, which might be attributed to their low reduction potential so that they were unable to effectively engage a single electron oxidation with 2a (entry 5). It was noteworthy that replacing the TFA by NaOAc, Cs2CO3, or K3PO4 completely inhibited this reaction, possibly due to the inertness of quinoline moiety under basic conditions (entry 6). Examination of a range of solvents showed that the reaction could be carried out efficiently in a mixed solvent of ACN and HFIP (entry 7), probably ascribed to the formation of hydrogen bond between N-centered radical and HFIP44,45,46. However, no product 3 was observed with HFIP or DMSO as sole solvent (entry 8). Other solvents such as DCE or DCM all gave 3 in lower yields (entry 9). Furthermore, the absence of Mes-Acr+ClO4-, [Co(dmgH)2Py]Cl, TFA, or blue light totally shut down the reaction (entry 10), demonstrating that each component plays a crucial role in promoting the reaction. Oxygen, as a green and abundant oxidant, has attracted our attention because it can not only act as an electron acceptor to promote the regeneration of photocatalysts47, but also as a hydrogen acceptor in dehydrogenation coupling reactions48. We believed that replacing cobaloxime catalyst with oxygen would also achieve this goal. Unfortunately, only a trace amount of 3 was detected when the reaction was conducted under an oxygen atmosphere without the addition of [Co(dmgH)2Py]Cl (entry 11). Finally, a survey of common N-protecting groups on amine substrates revealed 4-methoxybenzenesulfonyl as being optimal (entries 12-14).
Substrate scope
Having established the viable catalyst system and conditions, we turned our attention to investigate the generality of N-heteroarenes 1 using 4-methoxy-N-pentylbenzenesulfonamide 2a as a characteristic counterpart (Fig. 2). Quinolines substituted at the C2 or C4 positions reacted smoothly to give C4 or C2 coupling products 4-13 in fair to high yields, respectively. The target product 13 can be readily transformed into 4-aminoquinoline, which could be served as an analog of active pharmaceutical ingredient for treating malarial49. Reactions of benzothiazole and its derivatives with various substituents proceeded smoothly with good regioselectivity to afford the desired products 14-23 in 48–74% yields regardless of their electronic properties and substitution patterns, showing good functional group tolerance in this cooperative catalysis. Some other medicinally important heterocycles, such as isoquinoline, quinazoline, and phthalazine, were all feasible substrates to give a variety of alkylated products 24-27 in moderate yields. It was noteworthy that monocyclic heteroarenes such as pyridine, thiazole, pyrazine, pyrimidine, and pyridazine were also compatible with this protocol, providing the monosubstituted coupling products 28-35 in 41-78% yields. Furthermore, fused polycyclic substrate acridine was successfully turned into the corresponding alky-coupled product 36 in good yield. Unexpectedly, 2-chloroquinoxaline failed to undergo this transformation, which might be due to its decomposition under the established reaction conditions. Gratifyingly, the target product 37 was isolated in acceptable yield with K2S2O8 as oxidant under irradiation of purple light for 24 hours. Interestingly, the reaction of chromone in such photocatalytic system would give the C2-alkyl substituted chromanone 38 in moderate yield.
Reaction conditions: heteroarenes 1 (0.2 mmol, 1.0 equiv), N-alkylsulfonamides 2a (0.4 mmol, 2.0 equiv), Mes-Acr+ClO4- (0.004 mmol, 2 mol%), [Co(dmgH)2Py]Cl (0.01 mmol, 5 mol%), TFA (0.4 mmol, 2.0 equiv), ACN/HFIP = 3:1 (2.0 mL, 0.1 M), 2 × 25 W blue LEDs (λ = 450−460 nm), room temperature, under a N2 atmosphere, 24 h. aK2S2O8 (0.4 mmol, 2.0 equiv), TFA (0.4 mmol, 2.0 equiv), ACN/H2O = 1:1 (2.0 mL, 0.1 M), 2 × 25 W purple LEDs (λ = 390−400 nm), room temperature, under a N2 atmosphere, 24 hours. PMP = 4-methoxybenzenesulfonyl.
We next explored the scope of N-alkylsulfonamides. As shown in Fig. 3a, a number of sulfonamides including both linear and cyclic amides were suitable substrates under the optimal conditions. Sulfonamides carrying simple linear alkyl chains provided δ- and ε- substituted regional isomers via 1,5-HAT and 1,6-HAT, wherein the elongation of the carbon chain was more conducive to the 1,5-HAT process, as demonstrated by the generation of products 39-42. Noticeably, products 43 and 44 were obtained with moderate yields in unique regioselectivity. Substrates substituted at the α-, β- and γ-positions of nitrogen proceeded smoothly and delivered good yields of the products 45-48. Besides, functional groups, including ester, halogen, carbamate, and terminal benzoate, were proven to be tolerable, as demonstrated by the formation of products 49-52. As expected, methylene C−H bonds of cyclic motifs could be successfully functionalized, affording the target products 53-55 in satisfactory yields. However, benzylic C−H bonds were not suitable under the current conditions, possibly due to the steric hindrance of phenyl moiety (please see Supplementary Fig. 2 for details). Moreover, tertiary methine-containing and terminal methyl substrates were also viable abstraction partners, as demonstrated by the formation of products 56-60, albeit the latter resulting in lower yield. Significantly, this method could be applied to the late-stage modification of complex natural products and drug derivatives (Fig. 4b). For instance, medicinally relevant heteroaromatic drugs, such as the core of Roflumilast (61), Voriconazole (62) and Etofibrate (63), could undergo alkylation modification efficiently. Derivatives of readily accessible natural products such as borneol, l-menthol, Diacetonefructose, and saccharin behaved well to converted to the corresponding products (64-67) in moderate to high yields. Besides, a class of well-known nonsteroidal anti-inflammatory drugs, including Ibuprofen and Fenbufen, could provide the desired products 68 and 69 in good yields after protecting their carboxyl groups. Additionally, Cyclandelate, Fenofibrate, and Celecoxib were all compatible under the established conditions, giving the desired products 70-72 in appreciable yields.
Reaction conditions: heteroarenes 1 (0.2 mmol, 1.0 equiv), alkanes (0.2 mL), HAT agent (0.04 mmol, 20 mol%), Mes-Acr+ClO4- (0.004 mmol, 2 mol%), [Co(dmgH)2Py]Cl (0.01 mmol, 5 mol%), TFA (0.4 mmol, 2.0 equiv), ACN/HFIP = 3:1 (2.0 mL), 2 × 25 W blue LEDs (λ = 450−460 nm), room temperature, under a N2 atmosphere, 24 hours. Ar1 6-chlorobenzo[d]thiazole, Ar2 2-phenylquinoline, Ar3 4-methylquinoline.
In light of these results, we wonder if other kinds of C(sp3)−H substrates could be applied in such photo/cobalt dual catalyzed cross dehydrogenation coupling reaction via an intermolecular HAT process with N-alkylsulfonamide as an exogenous HAT catalyst (Fig. 4). To our delight, some simple cyclic alkanes such as cyclopentane, cyclohexane, cyclooctane and cyclododecane, could be heteroarylated smoothly to give the desired products 73-76 in moderate yields. Besides, the ethereal compounds 77-81 could be obtained under the optimized conditions. It was worth noting that ring-opening reactions could occur through C−O bond cleavage with cyclic ethers as coupling partners, providing β-, δ- and ε-heteroarylated alcohols 82-85 in fair to good yields, which made this synergistic strategy more robust because these results were difficult to realize typically by dehydrogenation coupling between heteroarenes and free alcohols to the best of our knowledge. In addition, the functionalization of α-C(sp3)–H in amine and alcohol derivatives, and β-C(sp3)–H in ketone derivatives was also successful (products 86-88).
To further demonstrate the versatility of this catalytic platform for the functionalization of remote inert C(sp3)−H bonds, we examined the reaction in the context of a range of radical trapping reagents under otherwise standard conditions (Fig. 5). Consistent with the results of classical PCET process, remote carbon radicals could still be produced smoothly and then engage in a conjugate addition reaction with an electron-deficient olefin partner to furnish a C(sp3)−C(sp3) bond under the catalysis of Mes-Acr+ClO4-, as demonstrated by the formation of products 89-91. Likewise, the so-formed carbon radical could undergo coupling with di-tert-butyl azodiformate (DBAD) to forge a C(sp3)−N bond (product 92). Cyanides, azides and halides act as powerful synthons for organic chemistry due to their ability to feed into a variety of functional group interconversions. Therefore, the direct introduction of cyano, azide, and halogens into an unactive C(sp3)−H position is of great significance for drugs synthesis and modification. Delightfully, by using electron-deficient SOMO-philes, where a functional group is attached to an aryl sulfonyl group, we directly achieved inert C(sp3)−H bonds cyanation and azidation (products 93 and 94). Additionally, the bromination and fluorination of inert C(sp3)−H bonds has been proven feasible by using N-bromosuccinimide and Selectfluor as coupling partners, respectively, as a mixture of mono- and dihalogenated products in the ratios of 9:1 and 1:1 (products 95 and 96). Moreover, the strategy was further applied to the remote C(sp3)−H trifluoromethylthiolation in the presence of 2-((trifluoromethyl)thio)isoindoline-1,3-dione (product 97). While the group of Studer and Xie independently reported the deuteration of unactivated C(sp3)−H bonds with amides as N-centered radical precursors19,50, N-centered sulfonamidyl radical-triggered deuteration of unactivated C(sp3)−H bonds has not been reported to date. By introducing 1,2-diphenyldisulfane as a synergistic catalyst and D2O as deuterium source, we were able to perform precise monodeuteration of N-alkylsulfonamide in a yield of 80% (product 98).
Reaction conditions: 2a (0.2 mmol, 1.0 equiv), radical trapping reagents (0.6 mmol, 3.0 equiv), Mes-Acr+ClO4- (0.006 mmol, 3 mol%), ACN/HFIP = 9:1 (2.0 mL, 0.1 M), 2 × 25 W blue LEDs (λ = 450−460 nm), room temperature, under a N2 atmosphere, 24 h. aradical trapping reagents (0.4 mmol, 2.0 equiv), b1,2-diphenyldisulfane (0.04 mmol, 20 mol%), ACN/D2O = 9:1 (2.0 mL, 0.1 M).
Gram-scale synthesis and application of product
The scalability of the protocol was demonstrated by the gram-scale reaction performed on both in batch and continuous-flow conditions without significant erosion of the yield. The synthetic application of this protocol was further demonstrated by the versatile transformations of the resultant product 3. For instance, treatment with NaI/PhI(OAc)2 under light irradiation could smoothly convert the resultant product 3 into a pyrrolidine derivative 99 via the iodine-mediated oxidative HLF cyclization. In addition, the N−H bond of 3 was easily proceeding a nucleophilic substitution with 3-bromoprop-1-ene under basic reaction conditions to produce a masked compound 100 in a high yield of 83% (Fig. 6a).
See Supplementary Information for more detailed reaction conditions and descriptions, including: a Gram-scale experiments and product transformations. b Radical quenching experiments and EPR texts: spin-trapping experiments with DMPO. c Control and radical clock experiments. d K2S2O8-promoted remote heteroarylation. e Cyclovoltammetric experiments. f Fluorescence quenching studies. g Hydrogen evolution detection. Ar2 2-phenylquinoline, TEMPO 2,2,6,6-tetramethylpiperidine 1-oxyl, BHT 3,5-di-tert-4-butylhydroxytoluene, DMPO 5,5-dimethyl-1-pyrroline N-oxide.
Mechanistic investigations
A series of validation experiments were conducted to explore the reaction mechanism. We found that the reaction was significantly suppressed in the presence of 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO) and 3,5-di-tert-4-butylhydroxytoluene (BHT), and the δ-alkyl radical adducts 101 and 102 were respectively detected by HRMS, indicating the involvement of the radical species in this reaction. The formation of δ-alkyl radical was further confirmed by hyperfine structure spectrum analysis under EPR texts with 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a radical capture agent. Noticeably, the radical signal was only observed under light irradiation, emphasizing the indispensable role of light in triggering the reaction (Fig. 6b). The methylated substrate 2y failed to proceed with remote heteroarylation, supporting the hypothesis that HAT process was enabled by NCRs generated by N−H bond cleavage. In contrast, heteroarylation occurred at the α-position of nitrogen in methylated substrate 2y, delivering the amidoalkylation product 103 in a yield of 32%, which implied that this transformation might involve the generation of less hindered α-aminoalkyl radical via the oxidation/deprotonation of tertiary amines51,52. With this insight, the radical clock experiments were carried out both under optimal conditions and in the case of TEMPO. Accordingly, the resultant products 104 and 105 were successfully detected by HRMS via α-aminoalkyl radical formation followed by radical-triggered ring-opening of cyclopropanes. Meanwhile, both the resultant products (104 and 105) and the radical adducts (106 and 107) were detected by HRMS in the case of TEMPO (Fig. 6c). All these results supported the presence of α-aminoalkyl radical in the control and radical clock experiments. Based on the previous reports53, we assigned it to a sequential electrochemical-chemical event. Specifically, neutral amines 2y and 2z underwent single electron oxidation to form sulfonamidyl radical cations I and III, which triggered deprotonation occurred at the α-position to give C-centered radicals II and IV since the enhanced acidity of C(sp3)−H bonds adjacent to the nitrogen atom. These enlightened results enabled us to assure that sulfonamidyl radical cations have been formed via single electron transfer between N-alkylsulfonamides bearing free N−H bonds and excited photocatalyst, which then underwent N−H bonds cleavage to generate NCRs because the N−H bonds were more acidic than α-C−H bonds in such NCRs-triggered remote functionalization. However, the possibility that the sulfonamidyl radical cation directly mediates the intramolecular HAT cannot be ruled out54. To probe more details of the formation of NCRs, we performed the model reaction in the presence of K2S2O8, which is an efficient oxidant widely applied in Minisci alkylation (Fig. 6d)55,56,57,58. Pleasingly, the remote heteroarylation proceeds efficiently, presenting the product 3 in a yield of 74%. Indeed, the generation of NCR in this transformation involved a sequential ET/PT event as well, in which the single electron oxidation between SO4•− (Ep/2 = +2.5–3.0 V)59 and 4-methoxy-N-pentylbenzenesulfonamide 2a to produce sulfonamidyl radical cation was the key to trigger reaction (please see Supplementary Fig. 13 for more detailed descriptions about reaction mechanism)60. Cyclic voltammetry studies were next performed to provide more evidence that the generation of sulfonamidyl radical cations through single electron oxidation was thermodynamically feasible in our reaction system (Fig. 6e). In this case, the oxidation half-peak potential of 4-methoxy-N-pentylbenzenesulfonamide (2a, PG1) was observed at +1.95 V (vs SCE in ACN), but upon the alteration of N-protecting groups on amine substrates, the half-peak potential of PG4 (+2.23 V vs SCE in ACN) and PG11 (+2.11 V vs SCE in ACN) was increased, and no redox features were displayed between 0 and 3.0 V (PG3, PG5, PG6, PG7, and PG10), which indicated that only 4-methoxy-N-pentylbenzenesulfonamide 2a could productively undergo the single-electron transfer with excited acridine photocatalyst. Despite the single electron transfer being thermodynamically permissible, the reactivity of PG2 (1.89 V vs SCE in ACN) and PG9 (1.97 V vs SCE in ACN) was inferior to PG1. It was worth noting that the remote heteroarylation of PG8 (+1.93 V vs SCE in ACN) and PG12 (+1.82 V vs SCE in ACN) did not proceed as effectively as PG1, albeit with much lower oxidation half-peak potential. We attributed the latter to the much higher bond dissociation energy of amidyl N−H bond, so that the production of corresponding NCR through N−H bond cleavage was energetically unfavorable61,62. Regretfully, we cannot provide a reasonable explanation for the former situation. Furthermore, Stern-Volmer quenching experiments were carried out by varying concentrations of [Co(dmgH)2Py]Cl, 2-phenylquinoline 1a, and 4-methoxy-N-pentylbenzenesulfonamide 2a in the presence of the acridine photocatalyst (Fig. 6f). It was found that the excited photocatalyst was not quenched by 1a. On the other hand, both [Co(dmgH)2Py]Cl and 2a could quench the fluorescence of photo-excited Mes-Acr+ClO4-, respectively. The quenching rates being directly proportional to their concentrations, which indicated the existence of single electron transfer between excited-state photocatalyst and [Co(dmgH)2Py]Cl or 2a. Considering the concentration of 2a is much higher than Co catalyst, this reaction is preferentially initiated by the generation of reductive state of photocatalyst from the excited state of photocatalyst via reductive quenching with N-alkylsulfonamides, whereas the Stern–Volmer quenching constant of [Co(dmgH)2Py]Cl was slightly greater than that of the 2a. Moreover, the hydrogen evolution was detected by GC-TCD analysis (Fig. 6g, please see Supplementary Fig. 31 for more details). Lastly, the light on-and-off experiment showed that continuous irradiation was essential for the product formation (please see Supplementary Fig. 32 for details).
DFT studies
To better understand and validate this mechanistic hypothesis, density functional theory (DFT) calculations using the PBE0 hybrid functional63 were performed in an investigation of the energetics of the proposed mechanism (Fig. 7). First, the 4-methoxy-N-pentylbenzenesulfonamide 1a is oxidized by the excited photocatalyst (PC+*) to form a sulfonamidyl radical cation Int1 via a SET, accompanied by the release of energy (4.6 kcal/mol). Subsequently, Int1 undergoes deprotonation to afford the sulfonamidyl radical Int2. This step is exergonic by 13.9 kcal/mol. In this stage, one molecular HFIP can form two strong hydrogen bonds with one N-centered radical Int2 to afford hydrogen-bonding complex Int3. Meanwhile, CoIII quenches the reduced photocatalyst (PC•) back to its ground state (PC+) with the release of energy (28.7 kcal/mol). These results suggest that both the stepwise ET/PT pathway and photocatalytic cycle are thermodynamically feasible. Later, a 1,5-HAT event proceeds via TS1, generating a C-centered radical Int4. This step is exergonic by 19.6 kcal/mol and has an 8.9 kcal/mol energy barrier. The radical intermediate Int4 then can attack C4 position of 1a-H+ to activate C(sp2)–H bond and giving the additional intermediate Int5, which is then rearomatized by CoII species through a barrier of 18.5 kcal/mol to afford the Int6 and Int7. The H2 evolution between Int6 and Int7 requires it to overcome a 23.6 kcal/mol energy barrier, and liberates the cobaloxime catalyst and product 3, which is identified as the rate-determining step. Noticeably, the process that Int7 accepts a proton provided by TFA to release H2 seems more favorable, proceeded with 19.3 kcal/mol energy barrier. The entire process of remote heteroarylation is exergonic by 12.9 kcal/mol. To reveal more details about the CoII-mediated rearomatisation of Int5, we further analyzed the spin density evolution during this step. It was found that the concerted TS3 shows a partial reduction of the spin density of CoII (0.60) (i.e., a partial cobalt oxidation from II to III), and reduction of the highly delocalized spin density in the Int5 to finally recover the aromaticity in the product, supporting that the rearomatisation was conducted by CoII species through hydrogen atom extraction from Int5 in an open-shell singlet transition state. Taken together, the bulk of these evidence supports the surmised stepwise ET/PT reaction pathways in remote C(sp3)−H functionalization. Finally, as a logical extension based on the current mechanistic framework, we believe that ET/PT mode will further enrich the development of unactive C(sp3)−H functionalization through the rational introduction of a radical trapping reagent or a cooperative catalyst that can match the oxidation potential of reductive state of acridine photocatalyst.
Discussion
In summary, we have developed a photoinduced stepwise ET/PT pathway for N-centered sulfonamidyl radical generation via N−H bond cleavage. And based on this protocol, we have achieved heteroarylation, alkylation, amination, cyanation, azidation, trifluoromethylthiolation, halogenation, and deuteration of unactivated C(sp3)−H bonds through NCRs-triggered 1,5-HAT. Performed under mild and redox-neutral conditions, this protocol is atom- and step-economical and obviates the need of noble metal catalysts and photocatalysts. The tolerance for diverse functional groups, as well as natural products and drug fragments, could make this approach attractive for complex molecule modification or late-stage functionalization. Further development of new photochemical remote functionalization is still under way in our lab. Moreover, a series of validation experiments and DFT calculations provide strong support for the proposed mechanism. Given the ubiquity and relevance of N-alkylsulfonamides in synthetic and medicinal chemistry, we believed that this photoredox-catalyzed unactivated C(sp3)−H bonds functionalization mechanism would be of conceptual and practical interest to chemists in both academic and industrial settings.
Methods
General procedure for remote C(sp3)–H heteroarylation of sulfonamides
To a 10 mL Schlenk tube equipped with a magnetic stirring bar was added heteroarene 1 (0.2 mmol), N-protected amines substrates 2 (0.4 mmol), Acr+-Mes-ClO4- (2 mol%) and Co(dmgH)2PyCl (5 mol%). After three cycles of evacuation and backfilling of the reaction flask with nitrogen, TFA (2.0 equiv.), ACN (1.5 mL) and HFIP (0.5 mL) were added to the tube under nitrogen. The mixture was then irradiated by two 25 W blue lamps for 24 h. The reaction mixture was quenched by adding 4 mL saturated NaHCO3 solution and 15 mL water and then extracted with ethyl acetate (3 × 20 mL). The combined organic extracts were washed by brine, dried over Na2SO4, filtered, concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel to afford the desired product 3-72.
General procedure for intermolecular dehydrogenative C(sp3)-H heteroarylation of alkanes
To a 10 mL Schlenk tube equipped with a magnetic stirring bar was added heteroarene 1 (0.2 mmol), Acr+-Mes-ClO4- (2 mol%), Co(dmgH)2PyCl (5 mol%) and N-(tert-butyl)−4-methoxybenzenesulfonamide (20 mol%). After three cycles of evacuation and backfilling of the reaction flask with nitrogen, TFA (2.0 equiv.), alkanes (0.2 mL), ACN (1.5 mL), and HFIP (0.5 mL) were added to the tube under nitrogen. The mixture was then irradiated by two 25 W blue lamps for 24 h. The reaction mixture was quenched by adding 4 mL saturated NaHCO3 solution and 15 mL water and then extracted with ethyl acetate (3 × 20 mL). The combined organic extracts were washed with brine, dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel to afford the desired product 73-88.
General procedure for remote C(sp3)–H functionalization of sulfonamides
To a 10 mL Schlenk tube equipped with a magnetic stirring bar was added 2a (0.2 mmol, 1.0 equiv.), radical trap reagent (0.4-0.6 mmol, 2.0-3.0 equiv.) and Acr+-Mes-ClO4- (3 mol%). After three cycles of evacuation and backfilling of the reaction flask with nitrogen, ACN (1.8 mL) and HFIP (0.2 mL) were added to the tube under nitrogen. The mixture was then irradiated by two 25 W blue lamps for 24 h. The reaction mixture was quenched by adding 15 mL water and then extracted with ethyl acetate (3 × 20 mL). The combined organic extracts were washed by brine, dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel to afford the desired product 89-97.
Data availability
All data are available from the corresponding author by request. The data generated or analyzed during the present study are included in this article and its Supplementary Information, including detailed information on experimental procedures, mechanistic studies, DFT calculations, compound characterization data and NMR spectra. The raw data of EPR tests, CV tests, Stern-Volmer fluorescence quenching tests and Cartesian coordinates in DFT calculations are available from the Source Data. Source data are provided with this paper.
References
Yi, H. et al. Recent advances in radical C–H activation/radical cross-coupling. Chem. Rev. 117, 9016–9085 (2017).
Cao, Z., Zhang, H., Wu, X., Li, Y. & Zhu, C. Radical heteroarylation of unactivated remote C(sp3)–H bonds via intramolecular heteroaryl migration. Org. Chem. Front. 8, 6395–6399 (2021).
Newhouse, T. & Baran, P. S. If C–H bonds could talk: selective C–H bond oxidation. Angew. Chem. Int. Ed. 50, 3362–3374 (2011).
Bergman, R. G. C–H activation. Nature 446, 391–393 (2007).
Font, M., Gulías, M. & Mascareñas, J. L. Transition-metal-catalyzed annulations involving the activation of C(sp3)−H bonds. Angew. Chem. Int. Ed. 61, e202112848 (2022).
Liu, B., Romine, A. M., Rubel, C. Z., Engle, K. M. & Shi, B.-F. Transition-metal-catalyzed, coordination-assisted functionalization of nonactivated C(sp3)–H bonds. Chem. Rev. 121, 14957–15074 (2021).
Zhang, Q. & Shi, B.-F. Site-selective functionalization of remote aliphatic C–H bonds via C–H metallation. Chem. Sci. 12, 841–852 (2021).
Holmberg-Douglas, N. & Nicewicz, D. A. Photoredox-catalyzed C–H functionalization reactions. Chem. Rev. 122, 1925–2016 (2022).
Wu, X., Ma, Z., Feng, T. & Zhu, C. Radical-mediated rearrangements: past, present, and future. Chem. Soc. Rev. 50, 11577–11613 (2021).
Chu, J. C. K. & Rovis, T. Complementary strategies for directed C(sp3)−H functionalization: a comparison of transition-metal-catalyzed activation, hydrogen atom transfer, and carbene/nitrene transfer. Angew. Chem. Int. Ed. 57, 62–101 (2018).
Guo, W., Wang, Q. & Zhu, J. Visible light photoredox-catalysed remote C–H functionalisation enabled by 1,5-hydrogen atom transfer (1,5-HAT). Chem. Soc. Rev. 50, 7359–7377 (2021).
Muñoz-Molina, J. M., Belderrain, T. R. & Pérez, P. J. Recent advances in copper-catalyzed radical C–H bond activation using N–F reagents. Synthesis 53, 51–64 (2021).
Tang, N., Wu, X. & Zhu, C. Practical, metal-free remote heteroarylation of amides via unactivated C(sp3)–H bond functionalization. Chem. Sci. 10, 6915–6919 (2019).
Bao, X., Wang, Q. & Zhu, J. Copper-catalyzed remote C(sp3)–H azidation and oxidative trifluoromethylation of benzohydrazides. Nat. Commun. 10, 769 (2019).
Munnuri, S. & Falck, J. R. Directed, remote dirhodium C(sp3)-H functionalization, desaturative annulation, and desaturation. J. Am. Chem. Soc. 144, 17989–17998 (2022).
Liu, H.-C. et al. Site-selective coupling of remote C(sp3)–H/meta-C(sp2)–H bonds enabled by Ru/photoredox dual catalysis and mechanistic studies. Chem. Sci. 13, 5382–5389 (2022).
Chen, H., Fan, W., Yuan, X.-A. & Yu, S. Site-selective remote C(sp3)–H heteroarylation of amides via organic photoredox catalysis. Nat. Commun. 10, 4743 (2019).
Chen, H. et al. Visible-light-driven intramolecular xanthylation of remote unactivated C(sp3)-H bonds. Green Synth. Catal. 4, 350–354 (2022).
Wang, L., Xia, Y., Derdau, V. & Studer, A. Remote site-selective radical C(sp3)−H monodeuteration of amides using D2O. Angew. Chem. Int. Ed. 60, 18645–18650 (2021).
Green, M. T., Dawson, J. H. & Gray, H. B. Oxoiron(IV) in chloroperoxidase compound II is basic: implications for P450 chemistry. Science 304, 1653–1656 (2004).
Bordwell, F. G., Zhang, S., Zhang, X.-M. & Liu, W.-Z. Homolytic bond dissociation enthalpies of the acidic H-A bonds caused by proximate substituents in sets of methyl ketones, carboxylic esters, and carboxamides related to changes in ground state energies. J. Am. Chem. Soc. 117, 7092–7096 (1995).
Murray, P. R. D. et al. Photochemical and electrochemical applications of proton-coupled electron transfer in organic synthesis. Chem. Rev. 122, 2017–2291 (2022).
Darcy, J. W., Koronkiewicz, B., Parada, G. A. & Mayer, J. M. A continuum of proton-coupled electron transfer reactivity. Acc. Chem. Res. 51, 2391–2399 (2018).
Gentry, E. C. & Knowles, R. R. Synthetic applications of proton-coupled electron transfer. Acc. Chem. Res. 49, 1546–1556 (2016).
Zhu, Q., Graff, D. E. & Knowles, R. R. Intermolecular anti-markovnikov hydroamination of unactivated alkenes with sulfonamides enabled by proton-coupled electron transfer. J. Am. Chem. Soc. 140, 741–747 (2018).
Choi, G. J., Zhu, Q., Miller, D. C., Gu, C. J. & Knowles, R. R. Catalytic alkylation of remote C–H bonds enabled by proton-coupled electron transfer. Nature 539, 268–271 (2016).
Chu, J. C. K. & Rovis, T. Amide-directed photoredox-catalysed C–C bond formation at unactivated sp3 C–H bonds. Nature 539, 272–275 (2016).
Chen, D.-F., Chu, J. C. K. & Rovis, T. Directed γ-C(sp3)–H alkylation of carboxylic acid derivatives through visible light photoredox catalysis. J. Am. Chem. Soc. 139, 14897–14900 (2017).
Thullen, S. M., Treacy, S. M. & Rovis, T. Regioselective alkylative cross-coupling of remote unactivated C(sp3)–H bonds. J. Am. Chem. Soc. 141, 14062–14067 (2019).
Xu, B. & Tambar, U. K. Remote allylation of unactivated C(sp3)–H bonds triggered by photogenerated amidyl radicals. ACS Catal. 9, 4627–4631 (2019).
Simons, R. T. et al. Directed photochemically mediated nickel-catalyzed (hetero)arylation of aliphatic C–H bonds. J. Am. Chem. Soc. 145, 3882–3890 (2023).
Deng, Z., Li, G.-X., He, G. & Chen, G. Photoredox-mediated remote C(sp3)–H heteroarylation of N-alkyl sulfonamides. J. Org. Chem. 84, 15777–15787 (2019).
Kwon, K., Simons, R. T., Nandakumar, M. & Roizen, J. L. Strategies to generate nitrogen-centered radicals that may rely on photoredox catalysis: development in reaction methodology and applications in organic synthesis. Chem. Rev. 122, 2353–2428 (2022).
Ravindar, L., Hasbullah, S. A., Hassan, N. I. & Qin, H.-L. Cross-coupling of C−H and N−H bonds: a hydrogen evolution strategy for the construction of C−N bonds. Eur. J. Org. Chem. 2022, e202200596 (2022).
Proctor, R. S. J. & Phipps, R. J. Recent advances in minisci-type reactions. Angew. Chem. Int. Ed. 58, 13666–13699 (2019).
Jeffrey, J. L., Terrett, J. A. & MacMillan, D. W. C. O–H hydrogen bonding promotes H-atom transfer from α C–H bonds for C-alkylation of alcohols. Science 349, 1532–1536 (2015).
Blanksby, S. J. & Ellison, G. B. Bond dissociation energies of organic molecules. Acc. Chem. Res. 36, 255–263 (2003).
Gao, J. et al. Photoredox/nickel dual catalysis-enabled cross-dehydrogenative C–H amination of indoles with unactivated amine. Org. Lett. 25, 7716–7720 (2023).
Wang, H., Gao, X., Lv, Z., Abdelilah, T. & Lei, A. Recent advances in oxidative R1-H/R2-H cross-coupling with hydrogen evolution via photo-/electrochemistry. Chem. Rev. 119, 6769–6787 (2019).
Chen, B., Wu, L.-Z. & Tung, C.-H. Photocatalytic activation of less reactive bonds and their functionalization via hydrogen-evolution cross-couplings. Acc. Chem. Res. 51, 2512–2523 (2018).
Schlegel, M., Qian, S. & Nicewicz, D. A. Aliphatic C–H functionalization using pyridine N-oxides as H-atom abstraction agents. ACS Catal. 12, 10499–10505 (2022).
Wang, B. et al. Photoinduced site-selective functionalization of aliphatic C–H bonds by pyridine N-oxide based HAT catalysts. ACS Catal. 12, 10441–10448 (2022).
Matsumoto, A., Yamamoto, M. & Maruoka, K. Cationic DABCO-based catalyst for site-selective C–H alkylation via photoinduced hydrogen-atom transfer. ACS Catal. 12, 2045–2051 (2022).
Dörr, M., Röckl, J. L., Rein, J., Schollmeyer, D. & Waldvogel, S. R. Electrochemical C−H functionalization of (hetero)arenes—optimized by DoE. Chem. Eur. J. 26, 10195–10198 (2020).
Berkessel, A., Adrio, J. A., Hüttenhain, D. & Neudörfl, J. M. Unveiling the “booster effect” of fluorinated alcohol solvents: aggregation-induced conformational changes and cooperatively enhanced H-bonding. J. Am. Chem. Soc. 128, 8421–8426 (2006).
Lucarini, M., Mugnaini, V., Pedulli, G. F. & Guerra, M. Hydrogen-bonding effects on the properties of phenoxyl radicals. An EPR, kinetic, and computational study. J. Am. Chem. Soc. 125, 8318–8329 (2003).
Liang, Z. et al. Photocatalytic dehydrogenated etherification of 2-aryl benzylic alcohols. Green Chem. 24, 7442–7447 (2022).
Zhou, J. et al. K2S2O8-catalyzed highly regioselective amidoalkylation of diverse N-heteroaromatics in water under visible light irradiation. Green Chem. 23, 5753–5758 (2021).
Schrezenmeier, E. & Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 16, 155–166 (2020).
Li, N. et al. Highly selective single and multiple deuteration of unactivated C(sp3)-H bonds. Nat. Commun. 13, 4224 (2022).
Shen, Y. & Rovis, T. Late-stage N-Me selective arylation of trialkylamines enabled by Ni/photoredox dual catalysis. J. Am. Chem. Soc. 143, 16364–16369 (2021).
Thullen, S. M. & Rovis, T. A mild hydroaminoalkylation of conjugated dienes using a unified cobalt and photoredox catalytic system. J. Am. Chem. Soc. 139, 15504–15508 (2017).
Nakajima, K., Miyake, Y. & Nishibayashi, Y. Synthetic utilization of α-aminoalkyl radicals and related species in visible light photoredox catalysis. Acc. Chem. Res. 49, 1946–1956 (2016).
Hofmann, A. W. Ueber die Einwirkung des Broms in alkalischer Lösung auf die Amine. Chem. Ber. 16, 558–560 (1883).
Quattrini, M. C. et al. Versatile cross-dehydrogenative coupling of heteroaromatics and hydrogen donors via decatungstate photocatalysis. Chem. Commun. 53, 2335–2338 (2017).
Garza-Sanchez, R. A., Tlahuext-Aca, A., Tavakoli, G. & Glorius, F. Visible light-mediated direct decarboxylative C–H functionalization of heteroarenes. ACS Catal. 7, 4057–4061 (2017).
McCallum, T., Jouanno, L.-A., Cannillo, A. & Barriault, L. Persulfate-enabled direct C–H alkylation of heteroarenes with unactivated ethers. Synlett 27, 1282–1286 (2016).
Kan, J., Huang, S., Lin, J., Zhang, M. & Su, W. Silver-catalyzed arylation of (hetero)arenes by oxidative decarboxylation of aromatic carboxylic acids. Angew. Chem. Int. Ed. 54, 2199–2203 (2015).
Mandal, S., Bera, T., Dubey, G., Saha, J. & Laha, J. K. Uses of K2S2O8 in metal-catalyzed and metal-free oxidative transformations. ACS Catal. 8, 5085–5144 (2018).
Zhang, H. et al. K2S2O8-induced site-selective phenoxazination/phenothiazination of electron-rich anilines. Green Chem. 24, 147–151 (2022).
Ashley, M. A. et al. Photoredox-catalyzed site-selective α-C(sp3)−H alkylation of primary amine derivatives. Angew. Chem. Int. Ed. 58, 4002–4006 (2019).
Šakić, D. & Zipse, H. Radical stability as a guideline in C–H amination reactions. Adv. Synth. Catal. 358, 3983–3991 (2016).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999).
Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (no. 22178314, J.L. and 21776254, J.L.). Furthermore, we thank Dr. Y. Weng for helpful discussions and Z. Liu from Shiyanjia Lab (www.shiyanjia.com) for the DFT calculations and GC-TCD analysis.
Author information
Authors and Affiliations
Contributions
J.L. designed and directed the project. C.W. supervised the work and wrote the manuscript. Z.C., J.S., L.T., W.W., and S.S. performed the experiments and mechanistic studies. All authors contributed to the analysis and interpretation of the data. C.W. and J.L. made manuscript revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, C., Chen, Z., Sun, J. et al. Sulfonamide-directed site-selective functionalization of unactivated C(sp3)−H enabled by photocatalytic sequential electron/proton transfer. Nat Commun 15, 5087 (2024). https://doi.org/10.1038/s41467-024-49337-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-49337-3
- Springer Nature Limited