Abstract
We present a homozygous missense mutation in the COL7A1 gene (NM_000094.4: c.6262G>A, p.G2088R) in a case of inversa recessive dystrophic epidermolysis bullosa (RDEB-I) from a nonconsanguineous Vietnamese family. Although a heterozygous form of this mutation in combination with a premature termination codon allele has been shown to cause RDEB-I, this is the first report of homozygosity of this mutation as the etiology. Here, we investigated the molecular basis of the patient’s disease for prenatal diagnosis after genetic counseling of the parents.
Similar content being viewed by others
Introduction
Epidermolysis bullosa (EB) is a group of disorders characterized by blisters and fragile skin formation. A decrease or lack of function of proteins involved in epidermal and dermal-epidermal adhesion in the skin basement membrane zone (BMZ) is found to be the cause of EB1. EB is often categorized into four major types, EB simplex, junctional EB, dystrophic EB, and Kindler’s syndrome, depending on the ultrastructural level of the BMZ in which the blisters form. Dystrophic EB mostly results from mutations in protein Col72,3. RDEB-I is a rare, autosomal recessive subtype of dystrophic EB. Symptoms may include neonatal generalized blisters in early childhood that may heal over the years; development of lesions of the oral, esophageal, anal, or genital mucosa; and transition of blisters from extremities to flexures and the body axis as patients age4,5.
The COL7A1 gene encoding Col7 is associated with RDEB-I. Col7 is the major component of the anchoring fibrils, attachment structures extending from the subbasal lamina of the dermoepidermal junction to the papillary dermis of the skin6. Col7 consists of three proα1 (VII) chains, each consisting of a central collagenous domain6. This is called the triple helix domain (THD), as it possesses Gly-X-Y patterned sequences that fold into a triple helix4,6. The THD contains 20 subdomains, numbered COL1 to COL20, with 19 interruptions in between, providing conformational flexibility to the THD4,7. Here, we present a case of a 9-year-old Vietnamese patient and his affected brother who expressed symptoms and molecular evidence of RDEB-I.
The study was approved by both the Institutional Review Board of the Institute of Genome Research, Vietnam Academy of Science and Technology, and the institutional review board of the Yokohama City University Faculty of Medicine. Informed consent was also obtained from the parents.
Genomic DNA was isolated from the whole blood of I.1, I.2, II.1, and II.2 and from amniotic fluid cells of II.3. Whole-exome sequencing (WES) was performed on proband II.1. The DNA library was prepared using the SureSelectXT Human All Exon 50 Mb, v5 libraries (Agilent Technologies, Santa Clara, CA, USA) and run on the 2500 platform (Illumina) with 101 bp paired-end reads (Illumina, USA). Quality-controlled reads were aligned to the human reference genome (UCSC hg19, NCBI build 37.1) using NovoAlign (http://www.novocraft.com/products/novoalign/). Polymerase chain reaction (PCR) duplications were removed using Picard (http://broadinstitute.github.io/picard/). Variants were called using the Genome Analysis Toolkit (GATK) (https://www.broadinstitute.org/gatk/index.php).
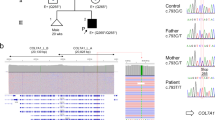
The proband exhibited blisters and erosions on the trunk and extremities, dystrophy of the toes, palm creases, toe fusion, and enamel hypoplasia (Fig. 1A–C). WES showed a homozygous missense mutation of COL7A1 (p.G2088R). Sanger results revealed that the proband’s affected brother was homozygous for the mutation, while his parents were heterozygous (p.G2088R) (Fig. 1D). Prenatal diagnosis for II.3 was performed using amniotic fluid cells, and a healthy brother was born with no pathogenic variants (Fig. 1F). Cluster Omega indicated the conservation of amino acid p.G2088 among humans and eight other species (Fig. 1E). The p.G2088R was predicted to be deleterious using in silico prediction tools (SIFT, Polyphen2, and MutationTaster).
Immunofluorescence mapping (IFM) was performed using damaged tissue samples collected from the proband’s forehead (rubbed skin) with antibodies against collagen IV, collagen VII, integrin α6, integrin β4, laminin γ2, and cytokeratin 5/6. The proband’s rubbed skin showed a similar collagen IV staining fluorescence signal as the control but a remarkable loss of signal in collagen VII staining (Fig. 2). This observation, coupled with the genetic testing of the proband’s family and clinical symptoms, supports a diagnosis of RDEB-I in the proband. A more detailed evaluation of the staining of six proteins is listed in Supplementary Table S1.
Immunofluorescence staining was performed with antibodies against collagen IV, collagen VII, integrin α6, integrin β4, laminin γ2, and cytokeratin 5/6. White arrows indicate the fluorescence signals of these proteins. The proband (rubbed skin sample) had a homozygous variant, p.G2088R, which was predicted to be deleterious by in silico prediction tools. Regarding collagen VII, while the control showed a broad and clear signal, the proband showed low and interrupted signals, indicating a significant reduction in collagen VII. However, the collagen IV signal of the proband was similar to that of the control. The proband sample presented a slight reduction in laminin γ2 and integrin β4, which are indicators for the diagnosis of junctional EB. The signals of integrin α6 and cytokeratin 5/6, which are indicators for the diagnosis of basal EB simplex and junctional EB, were similar to those of controls.
The proband’s mutation, p.G2088R, has previously been reported as the primary cause of RDEB-I. However, it is noteworthy that the previously reported mutation was compound heterozygous with a PTC mutation4,8. It was found in exon 75, subdomain COL12 of the Col7 THD domain4.
The Col7 protein consists of three proα1 (VII) chains, each with a central collagenous THD domain flanked by a larger NH2-terminal noncollagenous domain and a smaller COOH-terminal noncollagenous domain 4. The THD domain is composed of repeating Gly-X-Y sequences that fold into a triple-helical structure in which glycine is in the center of the helix, while the other two amino acids are on the surface4. Interstrand interactions between the three proα1 (VII) chains in triple helices occur between glycine and either the X or Y amino acid 9. The hydrogen bond N-H(Gly)⋅O = C(X) is the most significant interstrand bond responsible for the stability of the collagen structure, along with other potential hydrogen bonds9,10. Therefore, it is believed that glycine is more important than the X and Y amino acids in terms of structural stability, so the substitution of glycine would have more devastating effects on patients4,5. Moreover, glycine is a neutral, hydrophobic molecule, while arginine is a basic, hydrophilic molecule. The substitution of glycine with arginine could change the triple helix’s polarity, thereby further destabilizing the Col7 structure.
Four RDEB-I patients have been identified to have glycine substitutions, all near the border of the subdomains, suggesting that those glycine substitution locations may affect the pathological phenotype4. However, only 6/13 (46%) glycine substitutions of RDEB-I patients were located in either the first three or last three Gly-X-Y triplets, while the rest (54%) were in the central regions of the subdomains5.
Immunofluorescence results showed significantly reduced Col7 protein expression in the proband sample compared to the control sample (Fig. 2). This is strong evidence that the proband suffers from the dystrophic EB subtype. The signal was weak and discontinuous. This finding correlated with the proband’s homozygous mutation in the COL7A1 gene since we noticed a decrease in, not a total loss of, the proband’s collagen VII protein.
Type IV collagen was found above the blister and produced a normal signal, which fits the previous finding of similar IFM from a dystrophic EB patient1. However, reduced signal and slight discontinuities of laminin γ2 and integrin β4 were observed, corresponding to the interpretation of IFM results in patients with junctional EB1. This may call into question our diagnosis of RDEB-I in the proband. However, we should also consider the consequence of decreased Col7 expression in terms of the interaction between Col7 and these proteins, which are all localized to the same dermal - epidermal junctions. Moreover, the reductions in laminin γ2 and integrin β4 expression compared to the control levels were not as severe as those of Col7; thus, we suggest that experimental error and other variables should also be considered. Cytokeratin 5/6 antibodies showed slightly attenuated signals, and integrin α6 antibodies showed similar signals compared to those of the control. These antibodies are used as target proteins to diagnose basal EB simplex and junctional EB, so these results may support the hypothesis that our proband does not suffer from EB simplex or junctional EB1.
The previously reported cases of glycine substitution at p.G2088 are both compound heterozygous with a PTC mutation, to the best of our knowledge4,8. In patients with generalized RDEB - the most severe type of RDEB - homozygous PTC mutations in COL7A1 were often found, resulting in a total lack of Col78. Accordingly, heterozygous compound mutations with a PTC mutation and a codon substitution may have less severe phenotypes, resulting in milder symptoms and more localized blisters. Since our proband was homozygous for a codon substitution without any PTC mutation, it could be speculated that he should have reduced Col7 and even milder symptoms. However, he suffered from other quite serious symptoms, including nail dystrophies and enamel hypoplasia.
Since identical glycine substitution mutations caused both dominant and recessive inheritance, we are skeptical that underlying factors affect the mode of inheritance of EB11. Therefore, genetic evidence from our Vietnamese patient and his family may play an important role in supporting further studies of epidermolysis bullosa, because these data include all three allelic combinations of the mutation NM_000094.4: c.6262G>A, p.G2088R.
HGV Database
The relevant data from this Data Report are hosted at the Human Genome Variation Database at https://doi.org/10.6084/m9.figshare.hgv.3166.
Change history
06 June 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41439-022-00201-0
References
Has, C. & He, Y. Research techniques made simple: immunofluorescence antigen mapping in epidermolysis bullosa. J. Invest. Dermatol. 136, e65–e71 (2016).
Sakai, L. Y., Keene, D. R., Morris, N. P. & Burgeson, R. E. Type VII collagen is a major structural component of anchoring fibrils. J. Cell Biol. 103, 1577–1586 (1986).
Fine, J. D. Inherited epidermolysis bullosa. Orphanet J. Rare Dis. 5, 1–17 (2010).
Chiaverini, C. et al. Inversa dystrophic epidermolysis bullosa is caused by missense mutations at specific positions of the collagenic domain of collagen type VII. J. Invest. Dermatol. 130, 2508–2511 (2010).
Van Den Akker, P. C. et al. The inversa type of recessive dystrophic epidermolysis bullosa is caused by specific arginine and glycine substitutions in type VII collagen. J. Med. Genet. 48, 160–167 (2011).
Christiano, A. M., Greenspan, D. S., Lee, S. & Uitto, J. Cloning of human type VII collagen. Complete primary sequence of the α1(VII) chain and identification of intragenic polymorphisms. J. Biol. Chem. 269, 20256–20262 (1994).
Persikov, A. V. et al. Stability related bias in residues replacing glycines within the collagen triple helix (Gly-Xaa-Yaa) in inherited connective tissue disorders. Hum. Mutat. 24, 330–337 (2004).
Suzuki, S. et al. A case of recessive dystrophic epidermolysis bullosa caused by compound heterozygous mutations in the COL7A1 gene. Br. J. Dermatol 155, 838–840 (2006).
Shoulders, M. D. & Raines, R. T. Collagen structure and stability. Annu. Rev. Biochem. 78, 929–958 (2009).
Bella, J., Eaton, M., Brodsky, B. & Berman, H. M. Crystal and molecular structure, of a collagen-like peptide at 1.9 Å resolution. Science 266, 75–81 (1994).
Almaani, N. et al. Identical glycine substitution mutations in type VII collagen may underlie both dominant and recessive forms of dystrophic epidermolysis bullosa. Acta Derm. Venereol. 91, 262–266 (2011).
Acknowledgements
We thank Prof. Sumio Sugano for making this work possible. This work is supported in part by AMED under grant numbers JP21ek0109486, JP21ek0109549, and JP21ek0109493 (N.M.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Duong, N.T., Anh, L.T.L., Sau, N.H. et al. A rare homozygous missense mutation of COL7A1 in a Vietnamese family. Hum Genome Var 9, 13 (2022). https://doi.org/10.1038/s41439-022-00192-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41439-022-00192-y
- Springer Nature Limited