Abstract
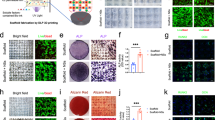
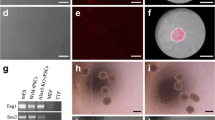
Cell therapy is a valuable strategy for the replacement of bone grafts and repair bone defects, and mesenchymal stem cells (MSCs) are the most frequently used cells. This study was designed to genetically edit MSCs to overexpress bone morphogenetic protein 9 (BMP-9) using Clustered Regularly Interspaced Short Palindromic Repeats/associated nuclease Cas9 (CRISPR-Cas9) technique to generate iMSCs-VPRBMP-9+, followed by in vitro evaluation of osteogenic potential and in vivo enhancement of bone formation in rat calvaria defects. Overexpression of BMP-9 was confirmed by its gene expression and protein expression, as well as its targets Hey-1, Bmpr1a, and Bmpr1b, Dlx-5, and Runx2 and protein expression of SMAD1/5/8 and pSMAD1/5/8. iMSCs-VPRBMP-9+ displayed significant changes in the expression of a panel of genes involved in TGF-β/BMP signaling pathway. As expected, overexpression of BMP-9 increased the osteogenic potential of MSCs indicated by increased gene expression of osteoblastic markers Runx2, Sp7, Alp, and Oc, higher ALP activity, and matrix mineralization. Rat calvarial bone defects treated with injection of iMSCs-VPRBMP-9+ exhibited increased bone formation and bone mineral density when compared with iMSCs-VPR- and phosphate buffered saline (PBS)-injected defects. This is the first study to confirm that CRISPR-edited MSCs overexpressing BMP-9 effectively enhance bone formation, providing novel options for exploring the capability of genetically edited cells to repair bone defects.




Similar content being viewed by others
Data availability
The corresponding author declares that the data are available if requested.
References
Brydone AS, Meek D, Maclaine S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc Inst Mech Eng H. 2010;224:1329–43. https://doi.org/10.1243/09544119JEIM770
Walmsley GG, Ransom RC, Zielins ER, Leavitt T, Flacco JS, Hu MS, et al. Stem cells in bone regeneration. Stem Cell Rev Rep. 2016;12:524–9. https://doi.org/10.1007/s12015-016-9665-5
Souza ATP, Freitas GP, Lopes HB, Ferraz EP, Oliveira FS, Beloti MM, et al. Effect of cell therapy with allogeneic osteoblasts on bone repair of rat calvaria defects. Cytotherapy. 2018;20:1267–77. https://doi.org/10.1016/j.jcyt.2018.06.010
Freitas GP, Lopes HB, Souza ATP, Oliveira PGFP, Almeida ALG, Souza LEB, et al. Cell therapy: effect of locally injected mesenchymal stromal cells derived from bone marrow or adipose tissue on bone regeneration of rat calvarial defects. Sci Rep. 2019;9:13476 https://doi.org/10.1038/s41598-019-50067-6
Freitas GP, Lopes HB, Souza ATP, Oliveira PGFP, Almeida ALG, Coelho PG, et al. Effect of cell therapy with osteoblasts differentiated from bone marrow or adipose tissue stromal cells on bone repair. Regen Med. 2019;14:1107–19. https://doi.org/10.2217/rme-2019-0036
Souza ATP, Lopes HB, Freitas GP, Ferraz EP, Oliveira FS, Almeida ALG, et al. Role of embryonic origin on osteogenic potential and bone repair capacity of rat calvarial osteoblasts. J Bone Miner Metab. 2020;38:481–90. https://doi.org/10.1007/s00774-020-01090-5
Fukumoto T, Sperling JW, Sanyal A, Fitzsimmons JS, Reinholz GG, Conover CA, et al. Combined effects of insulin-like growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthritis Cartilage. 2003;11:55–64. https://doi.org/10.1053/joca.2002.0869
Samee M, Kasugai S, Kondo H, Ohya K, Shimokawa H, Kuroda S. Bone morphogenetic protein-2 (BMP-2) and vascular endothelial growth factor (VEGF) transfection to human periosteal cells enhances osteoblast differentiation and bone formation. J Pharmacol Sci. 2008;108:18–31. https://doi.org/10.1254/jphs.08036fp
Wang Q, Huang C, Xue M, Zhang X. Expression of endogenous BMP-2 in periosteal progenitor cells is essential for bone healing. Bone. 2011;48:524–32. https://doi.org/10.1016/j.bone.2010.10.178
Hah YS, Jun JS, Lee SG, Park BW, Kim DR, Kim UK, et al. Vascular endothelial growth factor stimulates osteoblastic differentiation of cultured human periosteal-derived cells expressing vascular endothelial growth factor receptors. Mol Biol Rep. 2011;38:1443–50. https://doi.org/10.1007/s11033-010-0249-1
Bonilla-Claudio M, Wang J, Bai Y, Klysik E, Selever J, Martin JF. Bmp signaling regulates a dose-dependent transcriptional program to control facial skeletal development. Development. 2012;139:709–19. https://doi.org/10.1242/dev.073197
Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts). J Tissue Eng Regen Med. 2008;2:1–13. https://doi.org/10.1002/term.63.
Luu HH, Song WX, Luo X, Manning D, Luo J, Deng ZL, et al. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25:665–77. https://doi.org/10.1002/jor.20359
Beederman M, Lamplot JD, Nan G, Wang J, Liu X, Yin L, et al. BMP signaling in mesenchymal stem cell differentiation and bone formation. J Biomed Sci Eng. 2013;6:32–52. https://doi.org/10.4236/jbise.2013.68A1004
Wang Y, Hong S, Li M, Zhang J, Bi Y, He Y, et al. Noggin resistance contributes to the potent osteogenic capability of BMP9 in mesenchymal stem cells. J Orthop Res. 2013;31:1796–803. https://doi.org/10.1002/jor.22427
Lauzon MA, Drevelle O, Daviau A, Faucheux N. Effects of BMP-9 and BMP-2 on the PI3K/Akt pathway in MC3T3-E1 preosteoblasts. Tissue Eng Part A. 2016;22:1075–85. https://doi.org/10.1089/ten.TEA.2016.0151
Shinohara Y, Nakamura T, Shirakata Y, Noguchi K. Bone healing capabilities of recombinant human bone morphogenetic protein-9 (rhBMP-9) with a chitosan or collagen carrier in rat calvarial defects. Dent Mater J. 2016;35:454–60. https://doi.org/10.4012/dmj.2015-242
Nakamura T, Shirakata Y, Shinohara Y, Miron RJ, Hasegawa-Nakamura K, Fujioka-Kobayashi M, et al. Comparison of the effects of recombinant human bone morphogenetic protein-2 and -9 on bone formation in rat calvarial critical-size defects. Clin Oral Investig. 2017;21:2671–9. https://doi.org/10.1007/s00784-017-2069-3
Fujioka-Kobayashi M, Abd El Raouf M, Saulacic N, Kobayashi E, Zhang Y, Schaller B, et al. Superior bone-inducing potential of rhBMP9 compared to rhBMP2. J Biomed Mater Res A. 2018;106:1561–74. https://doi.org/10.1002/jbm.a.36359
Zhang R, Li X, Liu Y, Gao X, Zhu T, Lu L. Acceleration of bone regeneration in critical-size defect using BMP-9-loaded nHA/ColI/MWCNTs scaffolds seeded with bone marrow mesenchymal stem cells. Biomed Res Int. 2019;2019:7343957 https://doi.org/10.1155/2019/7343957
Dumanian ZP, Tollemar V, Ye J, Lu M, Zhu Y, Liao J, et al. Repair of critical sized cranial defects with BMP9-transduced calvarial cells delivered in a thermoresponsive scaffold. PLoS ONE. 2017;12:e0172327 https://doi.org/10.1371/journal.pone.0172327
Nie L, Yang X, Duan L, Huang E, Pengfei Z, Luo W, et al. The healing of alveolar bone defects with novel bio-implants composed of Ad-BMP9-transfected rDFCs and CHA scaffolds. Sci Rep. 2017;7:6373 https://doi.org/10.1038/s41598-017-06548-7
Lee CS, Bishop ES, Dumanian Z, Zhao C, Song D, Zhang F, et al. Bone morphogenetic protein-9-stimulated adipocyte-derived mesenchymal progenitors entrapped in a thermoresponsive nanocomposite scaffold facilitate cranial defect repair. J Craniofac Surg. 2019;30:1915–9. https://doi.org/10.1097/SCS.0000000000005465
Aslan H, Zilberman Y, Arbeli V, Sheyn D, Matan Y, Liebergall M, et al. Nucleofection-based ex vivo nonviral gene delivery to human stem cells as a platform for tissue regeneration. Tissue Eng. 2006;12:877–89. https://doi.org/10.1089/ten.2006.12.877
Chavez A, Scheiman J, Vora S, Pruitt BW, Tuttle M, Iyer EPR, et al. Highly efficient Cas9-mediated transcriptional programming. Nat Methods. 2015;12:326–8. https://doi.org/10.1038/nmeth.3312
Ledford H. CRISPR: gene editing is just the beginning. Nature. 2016;531:156–9. https://doi.org/10.1038/531156a.
La Russa MF, Qi LS. The new state of the art: Cas9 for gene activation and repression. Mol Cell Biol.2015;35:3800–9. https://doi.org/10.1128/MCB.00512-15.
Freitas GP, Souza AT, Lopes HB, Trevisan RL, Oliveira FS, Fernandes RR. et al. Mesenchymal stromal cells derived from bone marrow and adipose tissue: isolation, culture, characterization and differentiation. Bio-protocol.2020;4:e3534. https://doi.org/10.21769/BioProtoc.3534.
Lopes HB, Freitas GP, Elias CN, Tye C, Stein JL, Stein GS. et al. Participation of integrin β3 in osteoblast differentiation induced by titanium with nano or microtopography. J Biomed Mater Res A.2019;107:1303–13. https://doi.org/10.1002/jbm.a.36643.
Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H. et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res.2013;28:2–17. https://doi.org/10.1002/jbmr.1805.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. https://doi.org/10.1080/14653240600855905
Rostovskaya M, Anastassiadis K. Differential expression of surface markers in mouse bone marrow mesenchymal stromal cell subpopulations with distinct lineage commitment. PLoS One. 2012;7:e51221. https://doi.org/10.1371/journal.pone.0051221
Lundberg AS, Hahn WC, Gupta P, Weinberg RA. Genes involved in senescence and immortalization. Curr Opin Cell Biol. 2000;12:705–9. https://doi.org/10.1016/s0955-0674(00)00155-1
Fridman AL, Tainsky MA. Critical pathways in cellular senescence and immortalization revealed by gene expression profiling. Oncogene. 2008;27:5975–87. https://doi.org/10.1038/onc.2008.213
Li JZ, Li H, Sasaki T, Holman D, Beres B, Dumont RJ, et al. Osteogenic potential of five different recombinant human bone morphogenetic protein adenoviral vectors in the rat. Gene Ther. 2003;10:1735–43. https://doi.org/10.1038/sj.gt.3302075
Sheyn D, Kimelman-Bleich N, Pelled G, Zilberman Y, Gazit D, Gazit Z. Ultrasound-based nonviral gene delivery induces bone formation in vivo. Gene Ther. 2008;15:257–66. https://doi.org/10.1038/sj.gt.3303070
Alden TD, Beres EJ, Laurent JS, Engh JA, Das S, London SD, et al. The use of bone morphogenetic protein gene therapy in craniofacial bone repair. J Craniofac Surg. 2000;11:24–30. https://doi.org/10.1097/00001665-200011010-00005
Dayoub H, Dumont RJ, Li JZ, Dumont AS, Hankins GR, Kallmes DF, et al. Human mesenchymal stem cells transduced with recombinant bone morphogenetic protein-9 adenovirus promote osteogenesis in rodents. Tissue Eng. 2003;9:347–56. https://doi.org/10.1089/107632703764664819
Kimelman-Bleich N, Pelled G, Zilberman Y, Kallai I, Mizrahi O, Tawackoli W, et al. Targeted gene-and-host progenitor cell therapy for nonunion bone fracture repair. Mol Ther. 2011;19:53–9. https://doi.org/10.1038/mt.2010.190.
Phillips JE, Gersbach CA, García AJ. Virus-based gene therapy strategies for bone regeneration. Biomaterials. 2007;28:211–29. https://doi.org/10.1016/j.biomaterials.2006.07.032
Feeley BT, Conduah AH, Sugiyama O, Krenek L, Chen IS, Lieberman JR. In vivo molecular imaging of adenoviral versus lentiviral gene therapy in two bone formation models. J Orthop Res. 2006;24:1709–21. https://doi.org/10.1002/jor.20229
Sharff KA, Song WX, Luo X, Tang N, Luo J, Chen J, et al. Hey1 basic helix-loop-helix protein plays an important role in mediating BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. J Biol Chem. 2009;284:649–59. https://doi.org/10.1074/jbc.M806389200
Luo J, Tang M, Huang J, He BC, Gao JL, Chen L, et al. TGFbeta/BMP type I receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic signaling in mesenchymal stem cells. J Biol Chem. 2010;285:29588–98. https://doi.org/10.1074/jbc.M110.130518
Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, et al. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:1–16. https://doi.org/10.1007/s11154-006-9001-5
Komori T. Regulation of proliferation, differentiation and functions of osteoblasts by Runx2. Int J Mol Sci. 2019;20:1694. https://doi.org/10.3390/ijms20071694
Lamplot JD, Qin J, Nan G, Wang J, Liu X, Yin L, et al. BMP9 signaling in stem cell differentiation and osteogenesis. Am J Stem Cells. 2013;2:1–21.
Mostafa S, Pakvasa M, Coalson E, Zhu A, Alverdy A, Castillo H, et al. The wonders of BMP9: From mesenchymal stem cell differentiation, angiogenesis, neurogenesis, tumorigenesis, and metabolism to regenerative medicine. Genes Dis. 2019;6:201–23. https://doi.org/10.1016/j.gendis.2019.07.003
Chen L, Zou X, Zhang RX, Pi CJ, Wu N, Yin LJ, et al. IGF1 potentiates BMP9-induced osteogenic differentiation in mesenchymal stem cells through the enhancement of BMP/Smad signaling. BMB Rep. 2016;49:122–7. https://doi.org/10.5483/bmbrep.2016.49.2.228
Gabbitas B, Canalis E. Bone morphogenetic protein-2 inhibits the synthesis of insulin-like growth factor-binding protein-5 in bone cell cultures. Endocrinology. 1995;136:2397–403. https://doi.org/10.1210/endo.136.6.7538461
Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–35. https://doi.org/10.1210/er.2002-0023
Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. https://doi.org/10.1016/s0092-8674(01)00622-5
Millán JL. The role of phosphatases in the initiation of skeletal mineralization. Calcif Tissue Int. 2013;93:299–306. https://doi.org/10.1007/s00223-012-9672-8
Lu S, Wang J, Ye J, Zou Y, Zhu Y, Wei Q, et al. Bone morphogenetic protein 9 (BMP9) induces effective bone formation from reversibly immortalized multipotent adipose-derived (iMAD) mesenchymal stem cells. Am J Transl Res. 2016;8:3710–30.
Khorsand B, Elangovan S, Hong L, Dewerth A, Kormann MS, Salem AK. A comparative study of the bone regenerative effect of chemically modified RNA encoding BMP-2 or BMP-9. AAPS J. 2017;19:438–46. https://doi.org/10.1208/s12248-016-0034-8
Almeida ALG, Freitas GP, Lopes HB, Gimenes R, Siessere S, Sousa LG, et al. Effect of stem cells combined with a polymer/ceramic membrane on osteoporotic bone repair. Braz Oral Res. 2019;33:e079. https://doi.org/10.1590/1807-3107BOR-2019.vol33.0079
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–86. https://doi.org/10.1002/jbmr.141
Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7:125 https://doi.org/10.1186/s13287-016-0363-7
Shao J, Zhang W, Yang T. Using mesenchymal stem cells as a therapy for bone regeneration and repairing. Biol Res. 2015;48:62 https://doi.org/10.1186/s40659-015-0053-4
Acknowledgements
This research was supported by São Paulo Research Foundation (FAPESP, grants # 2016/23850-8; 2017/12622-7; 2019/01346-4) and National Institutes of Health (NIH, grants # R01 AR039588 and R01 DE029311. The English language review was done by ENAGO (www.enago.com).
Author information
Authors and Affiliations
Contributions
GPF and HBL undertook the experiments, analyzed the data, and drafted the manuscript. ATPS, M.P.O.G., and GKQ performed the in vivo experiments, analyzed the data, and revised the manuscript. JG and CT performed the in vitro experiments, analyzed the data, and revised the manuscript. JLS, GSS, JBL, MMB, and ALR designed the study, analyzed the data, and drafted the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Freitas, G.P., Lopes, H.B., Souza, A.T.P. et al. Mesenchymal stem cells overexpressing BMP-9 by CRISPR-Cas9 present high in vitro osteogenic potential and enhance in vivo bone formation. Gene Ther 28, 748–759 (2021). https://doi.org/10.1038/s41434-021-00248-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-021-00248-8
- Springer Nature Limited
This article is cited by
-
Developing hydrogels for gene therapy and tissue engineering
Journal of Nanobiotechnology (2024)
-
Potential therapeutic strategies for osteoarthritis via CRISPR/Cas9 mediated gene editing
Reviews in Endocrine and Metabolic Disorders (2024)
-
Research progress of engineered mesenchymal stem cells and their derived exosomes and their application in autoimmune/inflammatory diseases
Stem Cell Research & Therapy (2023)
-
A Brief Overview of Global Trends in MSC-Based Cell Therapy
Stem Cell Reviews and Reports (2022)