Abstract
Heat shock proteins (HSPs) depletion and protein misfolding are important causes of hepatocyte death and liver regeneration disorder in liver injury. HSF2BP, as its name implies, is a binding protein of HSF2, but the specific role of HSF2BP in heat shock response (HSR) remains unknown. The aim of this study is to identify the role of HSF2BP in HSR and acute liver injury. In this study, we found that HSF2BP expression increased significantly within 24 h after APAP administration, and the trend was highly consistent with that of HSP70. hsf2bp-KO and hsf2bp-TG mouse models demonstrated HSF2BP reduced hepatocyte death, ameliorated inflammation, and improved liver function in APAP- or D-GalN/LPS- induced liver injury. Meanwhile, a significant increase of the survival rate was observed in hsf2bp-TG mice after APAP administration. Further studies showed that HSF2BP upregulated the expression of HSF2 and HSP70 and inhibited the activation of Jnk1/2 and P38 MAPK. Additionally, HSP70 siRNA pretreatment abolished the effect of HSF2BP on the MAPK pathway in APAP-treated hepatocytes. The results reveal that HSF2BP is a protective factor in acute liver injury, and the HSF2BP/HSP70/MAPK regulatory axis is crucial for the pathogenesis of liver injury. HSF2BP is a potential therapeutic target for liver injury.
Similar content being viewed by others
Introduction
Acute liver injury is a common clinical complication caused by a variety of factors, such as viral infection, improper drug use, excessive alcohol intake, and sepsis [1, 2]. Its severe form, acute liver failure, is a severe consequence of abrupt massive hepatocyte death, evolving over days or weeks to a lethal outcome, with a clinical mortality of 25–75% [2]. It is well known that the liver is an important organ for protein, lipid, and glucose metabolism and synthesizes most plasma proteins. Improper drug use, severe infection and other stimuli trigger the inflammatory cytokine storm, oxidative stress and mitochondrial dysfunction, resulting in protein misfolding and hepatocyte death [3,4,5].
Heat shock response (HSR) is an important protective mechanism of hepatocytes in harmful conditions [6]. Heat shock proteins (HSPs) are highly conserved stress proteins, promoting protein molecular folding and assembly, and refolding or removing misfolded proteins [7]. Plenty of studies have confirmed that overexpression of HSPs alleviates hepatocyte death and liver injury [8,9,10,11,12]. For example, overexpression of HSP72 in mouse livers alleviates multiple stress-induced liver injury, and sodium arsenite prevents ischemia-reperfusion injury by upregulating hepatocyte HSP70 expression [8, 10]. Additionally, HSPs play crucial roles in liver regeneration [13].
The MAPK family is important to signal transmitters from the cell surface to the nucleus, regulating cell proliferation and differentiation, cell stress, and apoptosis [14]. A large number of studies have confirmed that activation of MAPK is a key event in liver injury and liver regeneration [15,16,17]. Recent studies showed that HSPs regulated the activation of MAPK in liver diseases [10].
Heat shock factor 2 binding protein (HSF2BP) is initially isolated from the human testicular cDNA library, which can directly bind to HSF2 [18]. However, the role of HSF2BP in HSR is still unclear. Meanwhile, the role of HSF2BP in liver injury and repair remains unknown. Based on the above evidence, we hypothesized that HSF2BP alleviates liver injury by modulating the HSR. The aim of the present study is to clarify the role of HSF2BP in liver injury and its specific mechanisms.
Results
HSF2BP expression and HSR in liver injury
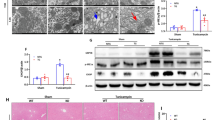
To investigate the changes in HSF2BP expression and HSR in liver injury, the mice and primary hepatocytes were administrated with APAP. We found that HSF2BP expression in liver sample were increased after APAP administration in mice (Fig. 1A, B). Quantitative results of HSR-associated proteins suggested that HSF2, HSF1, HSP90, HSP70, and HSP27 in the liver sample exhibited varying degrees of elevation after APAP administration at certain times, while HSP60 expression did not altered (Fig. 1A–C). Similar results were seen in primary hepatocytes. Compared with the PBS-administration, APAP-treated hepatocytes increased HSF2BP expression at 3, 6, 9, and 12 h, but not 24 h (Fig. 1D–F). The expression trends of HSF2BP and HSP70 were highly consistent. Immunohistochemistry showed that HSF2BP increased in livers after APAP treatment, especially at 6 h after administration (Fig. 1G, H). Moreover, a prominent increase in HSF2BP expression was observed in the livers of patients with the benign end-stage liver disease compared with the normal livers (Fig. S1). These results suggest that HSF2BP expression is increased in liver injury and is closely related to HSR.
Wild-type (WT) mice were intraperitoneally injected with 500 mg/kg APAP, and liver samples were harvested at 0, 3, 6, 9, 12, and 24 h after APAP administration. A–C Western blot analysis of the expression of HSF2BP, HSF2, HSF1, HSP90, HSP70, HSP60, and HSP27 in liver samples. *P < 0.05 versus the sham group; n = 6 per group. Primary hepatocytes were isolated and treated with 5 mM APAP. D–F Western blot analysis of the expression of HSF2BP, HSF2, HSF1, HSP90, HSP70, HSP60, and HSP27 at 0, 3, 6, 9, 12, and 24 h after APAP administration in hepatocytes. *P < 0.05 versus the sham group; n = 3 per group; G, H Immunohistochemistry analysis of liver HSF2BP expression. *P < 0.05 versus the sham group; n = 6 per group. mean ± SEM; The t-test was used to analyze the differences between the two groups.
Liver-specific hsf2bp gene deletion aggravates liver injury in mice
To further explore the role of HSF2BP in liver injury, liver-specific hsf2bp gene knockout mice were administrated with 500 mg/kg APAP or 700 mg/kg D-GalN + 10 μg/kg LPS. The histological results showed large necrosis and inflammatory cell infiltration in livers after APAP treatment, and the liver-specific hsf2bp−/− mice exhibited more severe liver injury than WT mice (Fig. 2A). Further quantitative analysis revealed that liver necrosis area and the histological score of hsf2bp−/− mice were higher than those of WT mice after APAP administration (Fig. 2B, C). Meanwhile, hsf2bp−/− mice showed higher serum ALT and AST levels than WT mice after APAP treatment at 3, 6, 12, and 24 h, respectively (Fig. 2D, E). Compared with the WT group, livers from the hsf2bp−/− mice exerted stronger positive TUNEL staining, suggesting HSF2BP plays an anti-apoptosis role in APAP-induced liver injury (Fig. 2F, G). Similar results were shown in the D-GalN/LPS-induced liver injury model. We observed more severe liver dysfunction in hsf2bp−/− mice than that in WT mice after D-GalN/LPS administration (Fig. 2H, I). Moreover, qPCR results suggested the liver tfn-α and cxcl-1 expression were higher in hsf2bp−/− mice than those in WT mice (Fig. 2J, K). These results confirmed that loss of HSF2BP aggravates liver injury.
WT mice and liver-specific hsf2bp−/− mice were intraperitoneally injected with 500 mg/kg APAP, and liver samples and blood samples were harvested at 0, 3, 6, 12, and 24 h after APAP administration. A Hematoxylin and eosin staining (H&E) of representative liver sections; B Percentage of necrotic areas; C Liver histological scores; D, E Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity; F, G TUNEL fluorescence staining (green), the corresponding nuclear counterstaining (blue) and percentage of TUNEL positive cells. WT and liver-specific hsf2bp−/− mice were intraperitoneally injected with 700 mg/kg D-GalN + 10 μg/kg LPS, and blood samples were harvested at 0 h and 6 h. H, I Serum ALT and AST activity. J, K qPCR analysis of Tnf-α and Cxcl-1 expression at 6 h after APAP administration. n = 6, mean ± SEM, *P < 0.05 versus WT group. The t-test was used to analyze the differences between the two groups.
Liver-specific HSF2BP overexpression alleviates liver injury in mice
We used liver-specific hsf2bp-TG mice overexpressing hsf2bp in hepatocytes to further investigate the function of HSF2BP in injury. Liver necrotic area, inflammatory cell infiltration, histological score, serum ALT and AST levels were all decreased in hsf2bp-TG mice compared with NTG mice (Fig. 3A–E). Meanwhile, 750 mg/kg APAP were administrated in hsf2bp-TG and NTG mice for survival analysis. A prominent increase in survival rate was observed in hsf2bp-TG mice compared with NTG mice after APAP treatment (Fig. 3F). TUNEL staining showed HSF2BP overexpression significantly reduced hepatocyte apoptosis at 6 and 24 h in APAP-induced liver injury (Fig. 3G, H). Consistent with the above results, hsf2bp-TG mice exhibited lower serum ALT and AST activity at 6 h after D-GalN/LPS administration (Fig. 3I, J). Additionally, qPCR results suggested the liver tfn-α and cxcl-1 expression were lower in hsf2bp-TG mice than those in NTG mice (Fig. 3K, L). Our results suggested that hsf2bp overexpression alleviates liver injury.
Liver-specific hsf2bp-TG mice and NTG mice were intraperitoneally injected with 500 mg/kg APAP, and liver samples and blood samples were harvested at 0, 3, 6, 12, and 24 h after APAP administration. A Hematoxylin and eosin staining (H&E) of representative liver sections; B Percentage of necrotic areas; C Liver histological scores; D, E Serum ALT and AST activity; F Liver-specific hsf2bp-TG mice and NTG mice were intraperitoneally injected with 750 mg/kg APAP. Survival analysis was conducted by Kaplan–Meier curves and log-rank testing. G, H TUNEL fluorescence staining (green), the corresponding nuclear counterstaining (blue) and percentage of TUNEL positive cells. WT mice and liver-specific hsf2bp-TG mice were intraperitoneally injected with 700 mg/kg D-GalN + 10 μg/kg LPS, and blood samples were harvested at 0 and 6 h. I, J Serum ALT and AST activity. K, L qPCR analysis of Tnf-α and Cxcl-1 expression at 6 h after APAP administration. n = 6, mean ± SEM, *P < 0.05 versus NTG group. The t-test was used to analyze the differences between the two groups.
HSF2BP upregulates HSF2/HSP70 signaling in APAP-treated mice
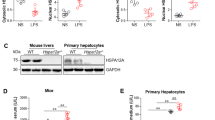
Liver-specific hsf2bp−/− mice and hsf2bp-TG mice were used to study the role of HSF2BP in HSR. We found that the expression of HSF2, HSF1, HSP90, HSP70, and HSP27 except HSP60, increased at 6 h after APAP treatment in liver samples. Meanwhile, HSF2 and HSP70 expression showed remarkable reductions in liver-specific hsf2bp−/− mice compared with the WT mice after APAP administration (Fig. 4A–C). Meanwhile, the expression of HSF1, HSP90, HSP60, and HSP27 showed no difference between WT mice and hsf2bp−/− mice. By contrast, hsf2bp-TG mice exhibited opposite results. A prominent increase in HSF2 and HSP70 expression was observed in liver-specific hsf2bp-TG mice compared with the NTG mice after APAP administration (Fig. 4D–F). Consistent with the western blot analysis, immunohistochemistry indicated that HSP70 expression was increased after APAP administration. Liver-specific hsf2bp gene deletion reduced liver HSP70 expression, while liver-specific HSF2BP overexpression increased liver HSP70 level at 6 h after APAP administration (Fig. 4G–J).
WT mice and liver-specific hsf2bp−/− mice were intraperitoneally injected with 500 mg/kg APAP, and liver samples were harvested at 0 and 6 h after APAP administration. A–C Western blot analysis of the expression of HSF2, HSF1, HSP90, HSP70, HSP60, and HSP27 in liver samples; *P < 0.05 versus the WT + APAP group. NTG mice and liver-specific hsf2bp-TG mice were intraperitoneally injected with 500 mg/kg APAP, and liver samples were harvested at 0 and 6 h after APAP administration. D–F Western blot analysis of the expression of HSF2, HSF1, HSP90, HSP70, HSP60, and HSP27 in liver samples. *P < 0.05 versus the NTG + APAP group. G, H Immunohistochemistry analysis of the liver HSF2BP expression in WT mice and liver-specific hsf2bp−/− mice; *P < 0.05 versus the WT + APAP group; I, J Immunohistochemistry analysis of the liver HSF2BP expression in NTG mice and liver-specific hsf2bp-TG mice; *P < 0.05 versus the NTG + APAP group; ns. means no difference, n = 6, mean ± SEM. The t-test was used to analyze the differences between the two groups.
HSF2BP restrains activation of Jnk and P38 MAPK in APAP-induced liver injury
MAPK pathway mediating the inflammatory response and cell death plays a crucial role in liver injury. We hypothesized that HSF2BP plays a protective role in liver injury by inhibition of MAPK phosphorylation. As reported in previous studies, APAP activated MAPK signaling, which is embodied in increased phosphorylation of Jnk1/2 (p-Jnk1/2), Erk1/2 (p-Erk1/2), P38 (p-P38), and Mek1/2 (p-Mek1/2) in mice. Meanwhile, MAPK activation-dependent kinase Mkk4 (p-Mkk4) and cJunS73 also increased after APAP administration (Fig. 5A–C). In contrast to the WT mice, liver-specific hsf2bp−/− mice increased p-Jnk1/2, p-P38, upstream kinase Mkk4 (p-Mkk4) and cJunS73, but not p-Erk1/2 and p-Mek1/2, in APAP-induced liver injury (Fig. 5A–C). However, hsf2bp-TG mice exhibited the opposite situation. Liver-specific hsf2bp overexpression abolished the increase of p-Jnk1/2, p-P38, upstream kinase Mkk4 (p-Mkk4) and cJunS73 in APAP-treated mice (Fig. 5D–F). Taken together, these results indicated that HSF2BP protects against liver injury via inhibition of phosphorylation of Jnk1/2 and P38.
WT mice and liver-specific hsf2bp−/− mice were intraperitoneally injected with 500 mg/kg APAP, and liver samples were harvested at 0 and 6 h after APAP administration. A–C Western blot analysis of the expression of Jnk1/2, p-Jnk1/2, Erk1/2, p-Erk1/2, P38, p-P38, T-Mek1/2, p-Mek1/2, p-MKK4, and p-cJunS73 in liver samples; *P < 0.05 versus the WT + APAP group. WT mice and liver-specific hsf2bp-TG mice were intraperitoneally injected with 500 mg/kg APAP, and liver samples were harvested at 0 and 6 h after APAP administration. D–F Western blot analysis of the expression of Jnk1/2, p-Jnk1/2, Erk1/2, p-Erk1/2, P38, p-P38, T-Mek1/2, p-Mek1/2, p-MKK4, and p-cJunS73 in liver samples. *P < 0.05 versus the NTG + APAP group. ns. means no difference, n = 6, mean ± SEM. The t-test was used to analyze the differences between the two groups.
HSF2BP regulates HSP70/MAPK pathway via binding to HSF2BP
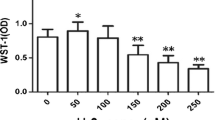
The foregoing results confirmed that HSF2BP was related to HSR, and we further determined the hepatocellular localization of HSF2BP and HSF2 by immunofluorescence. We found that under normal conditions, HSF2BP and HSF2 were distributed in the nucleus and cytoplasm (Fig. 6A). At 2 h after 5 mM APAP treatment, HSF2BP and HSF2 almost all entered the nucleus (Fig. 6A). Consistent with previous literature reports, the distribution of HSF2BP is highly consistent with that of HSF2, suggesting that HSF2BP can directly bind to HSF2 in APAP-induced hepatocyte injury and further regulate transcription of downstream HSPs. To further confirm whether HSF2BP inhibits MAPK phosphorylation via regulating HSP70 expression, we used small interfering RNA to interfere with HSP70 expression in hepatocytes. The results indicated that in HSP70 siRNA pretreated hepatocytes, the expression of p-Jnk1/2, p-P38, p-Mkk4 and cJunS73 showed no difference between the hsf2bp-TG hepatocyte and NTG hepatocyte after APAP administration (Fig. 6B–F). The deletion of HSP70 abolished the effect of HSF2BP on the MAPK pathway, indicating HSF2BP restrains activation of Jnk and P38 MAPK via upregulating HSP70 expression.
Primary hepatocytes were isolated and treated with 5 mM APAP. A immunofluorescent staining of HSF2 and HSF2BP at 6 h after APAP administration. Primary hepatocytes were pretreated with HSP70 siRNA. B–F Western blot analysis of the expression of Jnk1/2, p-Jnk1/2, P38, p-P38, p-MKK4, and p-cJunS73 in 5 mM APAP-treated hepatocytes. ns. means no difference, n = 6, mean ± SEM. The t-test was used to analyze the differences between the two groups. G HSF2BP protects against liver injury by regulating HSF2/HSP70/MAPK signaling.
Discussion
HSPs depletion and protein misfolding are important causes of hepatocyte death and liver regeneration disorder in liver injury [10, 13]. In this study, we found HSF2BP expression increased significantly within 24 h after APAP administration. Hepatocyte-specific hsf2bp-KO mice showed more severe hepatocyte death and inflammation and aggravated liver dysfunction in APAP and D-GalN/LPS-induced liver injury. Meanwhile, hepatocyte-specific hsf2bp-TG mice exhibited an opposite phenotype and had a higher survival rate. Further studies showed HSF2BP inhibited the activation of Jnk1/2 and P38 MAPK via upregulating the expression of HSP70 expression. HSF2BP/HSP70/MAPK regulatory axis is a potential therapeutic target for liver injury.
HSF2BP is a protein that can directly bind to HSF2, initially isolated from a human testis cDNA library by a yeast two-hybrid system [18]. Since its discovery in 1998, the role of HSF2BP is still poorly understood. The previous studies found that HSF2BP can also bind to BRCA2 and BNC1, playing different roles in cellular activity [19,20,21]. Current reports of HSF2BP mainly focus on meiosis, spermatogenesis and homologous recombination repair [22, 23]. Besides, HSF2BP has been reported to be transcribed in all cultured human cancer cell lines and elevated in some tumor samples [19, 24]. Inactivation of the hsf2bp gene in mice leads to male infertility [25]. This study found that the expression of HSF2BP was increased in livers and hepatocytes after APAP treatment both in vivo and in vitro study. In particular, the increase of HSF2BP mainly appeared at 6 and 12 h and then gradually decreased. At 24 h, there showed no difference compared with the control group in APAP-treated hepatocytes. Therefore, we guessed that the increase of HSF2BP within 24 h was compensatory, and it was gradual exhausted as APAP stimulation continues. More importantly, the increased expression of HSF2BP is highly consistent with that of HSP70, suggesting that HSF2BP may be involved in the HSR and have a close relationship with HSP70.
HSR is a defensive adaptive response characterized by changes in HSP gene expression, which promotes the folding and assembly of protein molecules, as well as the refolding or removal of misfolded proteins [7]. In recent years, abundant evidence proved that HSPs are closely related to liver injury [8, 10, 12, 26]. HSP70 family is the main intracellular HSP system, which plays a key role in protein transport and folding [27]. A recent study reported that HSP72, a member of the HSP70 family, is markedly increased in liver patients, and overexpression of HSP72 in mouse liver reduces multiple cause-induced liver injury [10]. Besides, sodium arsenite prevents ischemia-reperfusion injury by upregulating HSP70 expression in mouse liver [8]. HSF2BP, as its name implies, is a binding protein of HSF2, but the specific role of HSF2BP in HSR has not been reported. In this study, we constructed hsf2bp-KO and hsf2bp-TG mice and proved that HSF2BP reduced hepatocytes death, ameliorated inflammation, and improved liver function in APAP and D-GalN/LPS-induced liver injury. Meanwhile, we found HSF2BP upregulated the expression of HSF2 and HSP70, and HSP70 siRNA pretreatment abolished the effect of HSF2BP on the MAPK pathway in APAP-treated hepatocytes. The results indicated that HSF2BP protects against liver injury by regulating the HSP70-related HSR.
HSF1 and HSF2 are considered the most important heat shock factors (HSFs) [28]. Under normal conditions, HSF maintains a monomer structure in the cytoplasm. In response to heat shock or other forms of stress, HSF is assembled into trimers that enter the nucleus and binds to HSE located in the promoter region to promote the transcription of hsp mRNA [29]. Previous studies showed that HSF2 promotes the transcription of HSP70, but in the absence of HSF1, the transcription promoting effect of HSF2 on HSP70 disappears, suggesting that HSF2 and HSF1 may have a synergistic effect in the activation of HSP70 transcription [30,31,32,33]. In addition, the expression of HSF2 in the liver and heart is significantly higher than that in other tissues, suggesting HSF2 may play a non-negligible role in the stress response of the liver and heart [34]. In this study, we found that HSF2BP upregulated the expression of liver HSF2 and HSP70 in APAP-treated mice. Meanwhile, the distribution of HSF2BP is highly consistent with that of HSF2 in APAP-induced hepatocyte injury, suggesting that HSF2BP upregulated HSP70 expression via binding to the HSF2. However, whether HSF2BP regulates the HSP70 expression by participating in the interaction between HSF1 and HSF2 remains unclear and further studies are needed in the future.
MAPK family, including Erk1/2, Jnk1/2, and P38, are important signal transmitters from the cell surface to the nucleus, regulating cell proliferation and differentiation, cell stress, apoptosis, and other pathophysiological processes [14]. A large number of studies have confirmed that MAPK plays a crucial role in liver diseases [10, 16, 35]. For example, Jnk activation directly causes hepatocyte death during APAP-induced liver injury, and compound leflunomide attenuates APAP-induced liver injury by inhibition of Jnk phosphorylation [3, 36]. P38 MAPK is strongly activated by a variety of stress and plays important roles in inflammation and regulation of cell survival and differentiation [37]. In addition, an abundance of evidence proved that the MAPK pathway is regulated by HSPs [38, 39]. For example, HSP72 reduces cell death and oxidative stress in liver injury by inhibition of Jnk phosphorylation. In this study, we found that HSF2BP upregulated the expression of HSF2 and HSP70 and inhibited the activation of Jnk1/2 and P38 MAPK. HSP70 siRNA pretreatment abolished the effect of HSF2BP on the MAPK pathway in APAP-treated hepatocytes, suggesting HSF2BP protects against liver injury by regulating HSP70/MAPK signaling.
There are some limitations that should be mentioned in this study. Firstly, the present study is only based on animal experiments, and further clinical trials are needed. Secondly. we found that HSF2BP promoted the transcription of HSP70 via binding to HSF2. However, whether HSF2BP participates in the interaction between HSF1 and HSF2 needs further study. Finally, previous studies proved that HSF2BP can bind to HSF2, BRCA2, and BNC1 [19,20,21]. This study mainly focused on the role of HSF2BP in HSF2-related HSR, and other pathways need further study.
In conclusion, HSF2BP/HSP70/MAPK regulatory axis is crucial for the pathogenesis of acute liver injury. Targeting HSF2BP might be a novel strategy for the prevention or treatment of the acute liver injury.
Materials and methods
Detailed methods and full-length western blots are provided in the supplementary materials.
Experimental animals
In this study, all the animal experiments were performed on male wild-type C57BL/6 J mice, liver-specific hsf2bp-TG mice and liver-specific hsf2bp-KO mice (aged 6–8 weeks, weighing 20–25 g). Liver-specific hsf2bp-TG mice were constructed by inserting the ALB-Mouse Hsf2bp CDS-Polya fragment at Rosa26 on chromosome 6 with the CRISPR-Pro gene knockin technique. The hsf2bp-TG mice were identified by PCR with primer sequence: forward: 5′-GTTCACGGTCCATTATCAGTTG-3′, reverse: 5′-CACTGAAATGCTCAAATGGGAG-3′; Liver-specific hsf2bp-KO mice were constructed by CRISPR-Pro gene knockout technique and identified by PCR with primer sequence: forward: 5′-ACCATGTGGCAGTGAATATCCCAGA-3′, reverse: 5′-CCAGGGAAGGGAAGGAGTCTTCTA-3′. All experimental operations were performed in accordance with the requirements of the China Council on Animal Care and Use, and all experimental protocols were approved by the Ethics Committee of Xi’an Jiaotong University Health Science Center. In all experiments, mice were euthanized after anesthesia with isoflurane to minimize pain in this study.
Mouse models of liver injury
The liver failure model was established in mice by intraperitoneal injection of 500 mg/kg APAP (A105809, Aladdin, Shanghai, China) or 700 mg/kg D-GalN (G115553, Aladdin, Shanghai, China) + 10 μg/kg LPS (055:B5, L118716 Aladdin, Shanghai, China). In survival analysis, 750 mg/kg APAP were administrated in hsf2bp-TG and NTG mice for survival analysis.
Statistical analysis
All measurement data were expressed as the means ± standard error (SEM). The difference between groups was analyzed by t-test or one-way ANOVA. Kaplan–Meier curves and log-rank testing were used for survival analysis. All statistical analyses were performed with SPSS 18.0 software. p < 0.05 represented a significant difference.
Data availability
Data were available on request.
References
Rangaswamy C, Mailer RK, Englert H, Konrath S, Renne T. The contact system in liver injury. Semin Immunopathol. 2021;43:507–17.
Stravitz RT, Lee WM. Acute liver failure. Lancet. 2019;394:869–81.
Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59:583–94.
Franco-Gou R, Rosello-Catafau J, Casillas-Ramirez A, Massip-Salcedo M, Rimola A, Calvo N, et al. How ischaemic preconditioning protects small liver grafts. J Pathol. 2006;208:62–73.
Kucukoglu O, Guldiken N, Chen Y, Usachov V, El-Heliebi A, Haybaeck J, et al. High-fat diet triggers Mallory-Denk body formation through misfolding and crosslinking of excess keratin 8. Hepatology. 2014;60:169–78.
Liu Y, Yu M, Cui J, Du Y, Teng X, Zhang Z. Heat shock proteins took part in oxidative stress-mediated inflammatory injury via NF-kappaB pathway in excess manganese-treated chicken livers. Ecotoxicol Environ Saf. 2021;226:112833.
Gomez-Pastor R, Burchfiel ET, Thiele DJ. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat Rev Mol Cell Biol. 2018;19:4–19.
Kuboki S, Schuster R, Blanchard J, Pritts TA, Wong HR, Lentsch AB. Role of heat shock protein 70 in hepatic ischemia-reperfusion injury in mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1141–49.
Andoh H, Itoh H, Koyama K, Sato Y, Tashima Y. Heat shock protein 70 in rat liver with necrosis and regeneration induced by thioacetamide. J Gastroenterol. 1994;29:293–8.
Levada K, Guldiken N, Zhang X, Vella G, Mo FR, James LP, et al. Hsp72 protects against liver injury via attenuation of hepatocellular death, oxidative stress, and JNK signaling. J Hepatol. 2018;68:996–1005.
Terajima H, Enders G, Thiaener A, Hammer C, Kondo T, Thiery J, et al. Impact of hyperthermic preconditioning on postischemic hepatic microcirculatory disturbances in an isolated perfusion model of the rat liver. Hepatology. 2000;31:407–15.
Li L, Zhang T, Zhou L, Zhou L, Xing G, Chen Y, et al. Schisandrin B attenuates acetaminophen-induced hepatic injury through heat-shock protein 27 and 70 in mice. J Gastroenterol Hepatol. 2014;29:640–7.
Wolf JH, Bhatti TR, Fouraschen S, Chakravorty S, Wang L, Kurian S, et al. Heat shock protein 70 is required for optimal liver regeneration after partial hepatectomy in mice. Liver Transpl. 2014;20:376–85.
Rauch N, Rukhlenko OS, Kolch W, Kholodenko BN. MAPK kinase signalling dynamics regulate cell fate decisions and drug resistance. Curr Opin Struct Biol. 2016;41:151–8.
Li Z, Zhao Q, Lu Y, Zhang Y, Li L, Li M, et al. DDIT4 S-nitrosylation aids p38-MAPK signaling complex assembly to promote hepatic reactive oxygen species production. Adv Sci. 2021;8:e2101957.
Ocuin LM, Zeng S, Cavnar MJ, Sorenson EC, Bamboat ZM, Greer JB, et al. Nilotinib protects the murine liver from ischemia/reperfusion injury. J Hepatol. 2012;57:766–73.
Wang X, Mao W, Fang C, Tian S, Zhu X, Yang L, et al. Dusp14 protects against hepatic ischaemia-reperfusion injury via Tak1 suppression. J Hepatol. 2017;S0168–8278:32275–4.
Yoshima T, Yura T, Yanagi H. Novel testis-specific protein that interacts with heat shock factor 2. Gene. 1998;214:139–46.
Sato K, Brandsma I, van Rossum-Fikkert SE, Verkaik N, Oostra AB, Dorsman JC, et al. HSF2BP negatively regulates homologous recombination in DNA interstrand crosslink repair. Nucleic Acids Res. 2020;48:2442–56.
Brandsma I, Sato K, van Rossum-Fikkert SE, van Vliet N, Sleddens E, Reuter M, et al. HSF2BP interacts with a conserved domain of BRCA2 and is required for mouse spermatogenesis. Cell Rep. 2019;27:3790–8.e3797.
Wu Y, Liao S, Wang X, Wang S, Wang M, Han C. HSF2BP represses BNC1 transcriptional activity by sequestering BNC1 to the cytoplasm. FEBS Lett. 2013;587:2099–104.
Ghouil R, Miron S, Koornneef L, Veerman J, Paul MW, Le Du MH, et al. BRCA2 binding through a cryptic repeated motif to HSF2BP oligomers does not impact meiotic recombination. Nat Commun. 2021;12:4605.
Zhang J, Fujiwara Y, Yamamoto S, Shibuya H. A meiosis-specific BRCA2 binding protein recruits recombinases to DNA double-strand breaks to ensure homologous recombination. Nat Commun. 2019;10:722.
Huang Z, Liu Z, Cheng X, Han Z, Li J, Xia T, et al. Prognostic significance of HSF2BP in lung adenocarcinoma. Ann Transl Med. 2021;9:1559.
Felipe-Medina N, Caburet S, Sanchez-Saez F, Condezo YB, de Rooij DG, Gomez HL, et al. A missense in HSF2BP causing primary ovarian insufficiency affects meiotic recombination by its novel interactor C19ORF57/BRME1. Elife. 2020;9:e56996.
Liu J, Du S, Kong Q, Zhang X, Jiang S, Cao X, et al. HSPA12A attenuates lipopolysaccharide-induced liver injury through inhibiting caspase-11-mediated hepatocyte pyroptosis via PGC-1alpha-dependent acyloxyacyl hydrolase expression. Cell Death Differ. 2020;27:2651–67.
Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–32.
Westerheide SD, Raynes R, Powell C, Xue B, Uversky VN. HSF transcription factor family, heat shock response, and protein intrinsic disorder. Curr Protein Pept Sci. 2012;13:86–103.
Kurop MK, Huyen CM, Kelly JH, Blagg BSJ. The heat shock response and small molecule regulators. Eur J Med Chem. 2021;226:113846.
He H, Soncin F, Grammatikakis N, Li Y, Siganou A, Gong J, et al. Elevated expression of heat shock factor (HSF) 2A stimulates HSF1-induced transcription during stress. J Biol Chem. 2003;278:35465–75.
Jaeger AM, Pemble CWT, Sistonen L, Thiele DJ. Structures of HSF2 reveal mechanisms for differential regulation of human heat-shock factors. Nat Struct Mol Biol. 2016;23:147–54.
Korfanty J, Stokowy T, Widlak P, Gogler-Piglowska A, Handschuh L, Podkowinski J, et al. Crosstalk between HSF1 and HSF2 during the heat shock response in mouse testes. Int J Biochem Cell Biol. 2014;57:76–83.
Ostling P, Bjork JK, Roos-Mattjus P, Mezger V, Sistonen L. Heat shock factor 2 (HSF2) contributes to inducible expression of hsp genes through interplay with HSF1. J Biol Chem. 2007;282:7077–86.
Kim SS, Chang Z, Park JS. Identification, tissue distribution and characterization of two heat shock factors (HSFs) in goldfish (Carassius auratus). Fish Shellfish Immunol. 2015;43:375–86.
Renu K, Chakraborty R, Myakala H, Koti R, Famurewa AC, Madhyastha H, et al. Molecular mechanism of heavy metals (Lead, Chromium, Arsenic, Mercury, Nickel and Cadmium) - induced hepatotoxicity - A review. Chemosphere. 2021;271:129735.
Alamri RD, Elmeligy MA, Albalawi GA, Alquayr SM, Alsubhi SS, El-Ghaiesh SH. Leflunomide an immunomodulator with antineoplastic and antiviral potentials but drug-induced liver injury: a comprehensive review. Int Immunopharmacol. 2021;93:107398.
Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403–17.
Park KM, Kramers C, Vayssier-Taussat M, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury, MAPK and MAPK kinase activation, and inflammation by remote transient ureteral obstruction. J Biol Chem. 2002;277:2040–9.
Antonopoulou E, Kentepozidou E, Feidantsis K, Roufidou C, Despoti S, Chatzifotis S. Starvation and re-feeding affect Hsp expression, MAPK activation and antioxidant enzymes activity of European sea bass (Dicentrarchus labrax). Comp Biochem Physiol A Mol Integr Physiol. 2013;165:79–88.
Funding
This work was supported by the National Nature Science Foundation of China (No. 82172167 to RW and No. 82100654 to JB) and the Innovation Capacity Support Plan of Shaanxi Province (No. 2020TD-040 to RW).
Author information
Authors and Affiliations
Contributions
JB participated in the research design, performed most experiments, statistical analysis, and paper writing; JZ, TW, and MW participated in the animal studies and western blot analysis. WL participated in the ELISA and statistical analysis. YR and ZD participated in the cell culture and immunofluorescence. MK participated in the patient sample acquisition. SZ, ZW, and YL assisted with the design of the study. RW designed and supervised the study and revised the manuscript. All authors have read and agreed with the submission of the manuscript.
Corresponding author
Ethics declarations
Competing interests
RW, JZ, and JB are the inventor of a patent (ZL 202010490337.0, China) entitled “The application of HSF2BP in ischemia-reperfusion- or drug-induced liver injury”. This patent covers the fundamental concept of using HSF2BP as a therapeutic target for ischemia-reperfusion- or drug-induced liver injury.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Hans-Uwe Simon
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bi, J., Zhang, J., Ke, M. et al. HSF2BP protects against acute liver injury by regulating HSF2/HSP70/MAPK signaling in mice. Cell Death Dis 13, 830 (2022). https://doi.org/10.1038/s41419-022-05282-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-022-05282-x
- Springer Nature Limited
This article is cited by
-
The effects of rutin coat on the biodistribution and toxicities of iron oxide nanoparticles in rats
Journal of Nanoparticle Research (2024)
-
Overexpression of HSF2 binding protein suppresses endoplasmic reticulum stress via regulating subcellular localization of CDC73 in hepatocytes
Cell & Bioscience (2023)