Abstract
Background
Chemotherapy has limited efficacy in advanced digestive high-grade neuroendocrine neoplasms (HG-NEN) and prognosis is dismal. Predictive markers for palliative chemotherapy are lacking, and prognostic markers are limited.
Methods
Digestive HG-NEN patients (n = 229) were prospectively included 2013–2017. Pathological re-assessment revealed 188 neuroendocrine carcinomas (NEC) and 41 neuroendocrine tumours (NET G3). Tumour-DNA was sequenced across 360 cancer-related genes, assessing mutations (mut) and copy number alterations. We linked sequencing results to clinical information and explored potential markers for first-line chemotherapy efficacy and survival.
Results
In NEC given cis/carboplatin and etoposide (PE), TP53mut predicted inferior response rate in multivariate analyses (p = 0.009) and no BRAFmut NEC showed response. In overall assessment of PE-treated NEC, no genetic alterations were prognostic for OS. For small-cell NEC, TP53mut were associated with longer OS (p = 0.011) and RB1 deletions predicted lack of immediate-progression (p = 0.003). In non-small cell NEC, APC mut were associated with immediate-progression and shorter PFS (p = 0.008/p = 0.004). For NET G3, ATRXmut, ARID1A- and ERS1 deletions were associated with shorter PFS.
Conclusion
Correlations between genetic alterations and response/immediate-progression to PE were frequent in NEC but affected PFS or OS only when subdividing for cell-type. The classification of digestive NEC into large- and small-cell seems therefore molecularly and clinically relevant.
Similar content being viewed by others
Introduction
Neuroendocrine neoplasms (NEN) encompass a variety of neoplasms with different phenotypes, ranging from indolent low-grade neuroendocrine tumours (NET) to aggressive neuroendocrine carcinoma (NEC). The 2019 WHO classification of digestive high-grade NEN (HG-NEN) separated well-differentiated NET G3 from poorly differentiated NEC, both with a high proliferation defined by Ki-67 > 20% [1]. Digestive NET G3 and NEC entities differ in clinical and molecular characteristics, response to treatment and prognosis [2,3,4,5]. Metastatic NEC is associated with an especially poor prognosis, with median overall survival ranging from 1 month if untreated, to 11–12 months if given chemotherapy [6,7,8]. Established first-line palliative chemotherapy consisting of cis/carboplatin and etoposide is extrapolated from treatment of small-cell lung cancer (SCLC) based on clinical and morphological similarities [9,10,11]. The treatment has been unchanged since the 1990s, as has the dismal prognosis. Up to 30% of digestive NEC have an immediate disease progression evident at first radiological evaluation after receiving cis/carboplatin and etoposide. Progression free survival (PFS) is only 4–5 months. Metastatic NET G3 have a better prognosis compared to NEC, with a median overall survival (OS) of 33–36 months, although the efficacy of cis/carboplatin and etoposide has been reported inferior [4, 5, 12].
The implementation of next generation sequencing has led to a changed paradigm of cancer treatment by identifying genetic alterations targetable for treatments, with great success across a variety of cancers. Such molecular aberrations have also shown to be of value as predictors of treatment effect [13]. In a recent comprehensive molecular overview assessing digestive HG-NEN with a targeted approach covering 360 genes, we reported differences in both mutational frequency and genes affected when comparing NEC and NET G3 and a clear difference compared to previous reports on SCLC. In the same study, we found potentially targetable aberrations in 66% of 152 included NEC [3]. In a whole genome sequencing approach examining 16 NEC, 94% were reported to have potential targetable molecular alterations [14]. To date, few studies have assessed the clinical impact of molecular alterations in NEC [15, 16]. Thus, reliable biomarkers to guide treatment choice and to predict efficacy of therapy and survival for digestive HG-NEN are lacking. The aim of the present study was to evaluate the impact of genetic alterations on treatment outcomes and survival in a large cohort of digestive HG-NEN. In addition, through identification of potential molecular drivers among digestive HG-NEN, we aimed to elucidate potential targets eligible for future targeted treatment approaches.
Methods
Patients
Patients diagnosed with HG-NEN with digestive primary, or unknown primary with a predominance of gastrointestinal metastases were included prospectively from 2013–2017 from nine Scandinavian secondary and tertiary hospitals. Clinical data were available through the Nordic NEC Registry. Patients (N = 180) from our recent study on molecular characteristics of digestive HG-NEN were included. These patients were selected based on available tumour and matched normal tissue, allowing for the analysis of somatic copy number alterations (CNA) [3]. In addition, 49 novel digestive HG-NEN cases without matched normal tissue were included. As such, 229 patients were included in the present analyses. Formalin-Fixed-Paraffin-Embedded tumour tissue and tumour sections (haematoxylin/eosin, synaptophysin, chromogranin A and Ki-67) were collected for all patients. Patients were enroled prior to the formal introduction of NET G3 among digestive HG-NEN [1]. To ensure correct stratification according to the 2019 WHO classification, all cases were blinded and re-evaluated digitally in 2021-22 by three experienced NEN pathologists (AP, AC and IMBL). Ki-67 was recalculated. Initial ambiguous cases were discussed and decided on in a consensus meeting. Best response was reported according to radiological evaluation by RECIST criteria v 1.1. Mixed Neuroendocrine Non-Neuroendocrine tumours (MiNEN) were excluded (MiNEN covering both small cell NEC/adenocarcinomas and large cell NEC/adenocarcinomas). As the aggressiveness of NEC could prohibit more than one line of treatment, we assessed both immediate progression at first evaluation and response rate (RR) as endpoints assessing treatment efficacy. RR was defined as the proportion of responders (patients with complete or partial response). Immediate progression was defined as the proportion of patients with progression at first evaluation after initiation of first-line chemotherapy. In addition to radiological RECIST progression, three patients with confirmed clinical progression within 2 months of first course of chemotherapy (radiology not performed) were included in the immediate progression group.
Molecular analyses
Tissue collection, MSI analyses, DNA isolation, library preparation, sequencing, data processing and bioinformatical analyses were performed as described previously [3]. In brief the sequencing analyses covered the coding regions of a targeted panel of 360 cancer related genes. For the 49 cases without normal tissue for filtering of germline variants, somatic mutation calling was restricted to canonical driver mutations, identified following a pre-planned approach as previously described [17]. MSI analyses were available for 180 included patients. To avoid cases with potentially false low TMB, we included only cases with normal tissue for comparison (N = 180).
Statistics
Categorical variables were presented as percentages and continuous variables as median/means and range, as appropriate. For direct group comparisons, Chi-squared test was applied. The predictive values of categorical variables were explored using logistic regression analyses. PFS was calculated from the start of first-line chemotherapy to the date of progression or last known follow-up. Overall survival was, for the whole unselected cohort (n = 229), calculated from the date of diagnosis of high-grade NEN and for those with advanced disease (n = 206), from date of metastases/non-resectable disease, both to date of death or last follow-up. Survival curves were estimated using the Kaplan-Meier method, with log rank test for statistical significance. For estimation of hazard ratio, the cox regression model was used. Variables with a p < 0.10 in univariate analyses or assumed of particular potential clinical value (the majority pre-defined) were included in multivariate models. As BRAF mutated NEC had a zero-response rate to cis/carboplatin and etoposide, this mutation could not be included in the MVA. PS is known as an extremely strong prognostic factor for NEC, and we therefore only analysed cases with PS < 2. P values are reported as two-sided and values <0.05 were considered statistically significant. If not otherwise specified, all analyses for NEC were done on the subgroup receiving cis/carboplatin and etoposide (N = 123; Table 1), whereas for NET G3, for all chemotherapy regimens combined. The selected genetic alterations in our analyses were predefined as the top 10 alterations. Multiple testing was assessed by Benjamini-Hochberg procedure. Statistical analyses were performed using STATA version 16.1.
Results
Baseline characteristics
After pathological re-evaluation of 229 NEN, 188 (82%) were classified as NEC and 41 (18%) as NET G3. Pancreas was the most common primary tumour site for NET G3, while colorectal primary was most common for NEC. Baseline characteristics are summarised in Table 1. Out of the 229 included patients, 23 underwent curative surgery without evidence of residual disease throughout a median follow-up time of 53.7 months. Of the 207 cases with advanced disease, six had localised non-resectable (NR) disease and 201 had metastatic disease. Further out of these 207, one patient underwent curative surgery, 176 received first-line palliative chemotherapy, 11 radiotherapy, two radio-nucleotide treatment and 17 best supportive care. The majority of NEC were treated with cis/carboplatin and etoposide as first-line palliative chemotherapy (N = 123; 80%). For NET G3, 13 (46%) received first-line treatment with cis/carboplatin and etoposide and 10 (36%) with capecitabine/temozolomide.
Response and survival according to primary NEC site, NEC cell type and differentiation (NET G3 vs NEC)
For NEC given first-line cis/carboplatin and etoposide, RR was 37% whereas 35% had immediate progression at first evaluation after initiation of treatment. RR was 9% among colon NEC (2/22), significantly inferior to a 44% RR for extra-colonic NEC (39/88; p = 0.002). RR was better for small-cell NEC (SC-NEC) compared to large cell NEC (LC-NEC): 48 vs 26% p = 0.019. We found no significant difference in RR to cis/carboplatin and etoposide comparing NET G3 (N = 12) and NEC (N = 110; 37 vs 42%, p = 0.766).
We found no difference in PFS depending on primary NEC site, comparing colon to extra-colonic (2.1 vs 4.1 m, p = 0.170) or pancreatic to non-pancreatic (3.3 vs 5.6 m, p = 0.076). For patients given cis/carboplatin and etoposide there were no difference in PFS comparing SC- and LC-NEC (4.9 vs 2.4 m, p = 0.076), nor comparing NET G3 and NEC (3.6 vs 3.3 m, p = 0.577).
Assessing the whole cohort, we found a significant longer OS for NET G3 compared to NEC (22.2 vs 8 m, p < 0.001). The difference between NET G3 and NEC was significant for those given chemotherapy in general (18.6 vs 8.5 m, p < 0.001) but not for those given cis/carboplatin and etoposide (11.1 vs 8.8 m, p = 0.143). We found no difference in OS after cis/ carboplatin and etoposide when comparing SC and LC-NEC (9.2 vs 8.5 m, p = 0.53), nor when comparing colon to extra-colonic NEC (8.4 vs 8.9 m, p = 0.743) or pancreatic to non- pancreatic NEC (11.1 vs 8.7 m, p = 0.151).
Response and survival according to clinical biomarkers
For NEC patients given cis/carboplatin and etoposide, performance status (PS) did not influence RR, but PFS was longer with PS 0–1 compared to PS ≥ 2 (4.1 vs 2.2 m, p = 0.004), as were OS (10.2 vs 4.7 m, p < 0.001). In a univariate model, significantly inferior OS for NEC was seen for CRP > 10 (7.4 vs 11 m, p = 0.047) and elevated lactate dehydrogenase (LDH) (6.3 vs 11.1 m, p = 0.032), but without affecting RR or PFS.
Genetic alterations and treatment outcome in NEC
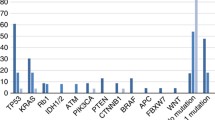
The top-ten genetic most frequent alterations for NEC cases are shown in Fig. 1. Outcomes dependent on genetic alterations for NEC given cis/carboplatin and etoposide are summarised in Table 2. NEC with TP53 mutations had a numerically lower RR than TP53 wild-type cases (47 vs 31%, p = 0.108). We observed no responders among BRAF V600E mutated NEC (n = 9), compared to 41% among BRAF wild type (n = 101; p = 0.016) and double-wild type BRAF/KRAS had higher RR (45 vs 22%, p = 0.023) compared to NEC harbouring either mutation. No detrimental impact of BRAF status on OS was found. CNA did not significantly affect treatment outcome, but cases with MYC amplification had a numerically higher rate of immediate progression at first evaluation after initiation of chemotherapy (40 vs 23%, p = 0.099). For pancreatic NEC, neither RB1 deletions nor TP53 mutation affected response to cis/carboplatin and etoposide. We found a significantly higher rate of immediate progression in rectal NEC with ARID1A deletions (56 vs 33%, OR 6.43, 95%CI 1.05–39.33, p = 0.044) and a strong trend towards inferior RR (OR 0.19, 95%CI 0.03–1.02, p = 0.052).
We found no significant correlation between genetic alterations and PFS as PFS was short regardless of any alteration. No significant correlation was found between genetic alterations and overall survival. There was a non-significant trend in univariate analyses towards longer survival for those harbouring TP53 mutation 10.2 vs 7.7 m (p = 0.068). Tumour mutation burden (TMB) was generally low, somewhat higher for NEC (5.0) than for NET G3 (3.2). Comparing NEC cases above vs below mean TMB, we found no difference in RR, PFS or OS after cis/carboplatin and etoposide. 9/10 NEC cases with TMB ≥ 10 had MSI.
Genetic alterations and treatment outcome according to NEC cell type
For SC-NEC given cis/carboplatin and etoposide, RB1 deletion predicted disease control (0% immediate progression vs 38%, p = 0.003) and TP53 mutation was associated with longer OS (10.2 vs 7.3 m, p = 0.011). For LC-NEC, TP53 mutated cases had lower RR (16 vs 43%, p = 0.033) whereas APC mutated cases had higher rate of immediate progression (77 vs 32%, p = 0.008) and shorter PFS (3.3 vs 1.8 m, p = 0.004). ARID1A deleted LC-NEC cases had longer PFS (5.0 vs 2.1 months, p = 0.032). Significant findings are illustrated in Fig. 2a–d, whereas Supplementary Table 1 summarise all outcomes for cis/carboplatin and etoposide for NEC dependent on cell type. In the MVA, TP53 mutation and colon primary were significant predictors for inferior RR to cis/carboplatin and etoposide (p = 0.008 and 0.007 respectively; Table 3a). Only elevated LDH correlated with inferior survival (Table 3b).
a Response to cis/carboplatin and etoposide according to TP53 status for LC-NEC (N = 61). b Immediate progression to cis/carboplatin and etoposide according to APC status for LC-NEC (N = 61). c Progression free survival (PFS) after cis/carboplatin and etoposide for LC-NEC according to APC status (N = 61). d Overall survival (OS) for SC-NEC according to TP53 status (N = 61).
NET G3
When assessing NET G3 for treatment outcome related to genetic alterations, we found no correlation to RR. MEN1 mutation predicted immediate progression (p = 0.042), however only four cases (10%) harboured such a mutation. Shorter PFS was associated with ARID1A deletion (3.6 vs 12.6 m, p = 0.029), ESR1 deletions (3.3. vs 9.8 m, p = 0.012) and ATRX mutation (2.6 vs 7.4 m, p = 0.022). After first-line chemotherapy, there was a trend towards longer survival for those without ESR1 deletion (22.2 vs 8.3 m, p = 0.068). The most frequent genetic alterations for NET G3 are shown in Supplementary Fig. 1. Other factors affecting OS after chemotherapy were elevated LDH (8.3 vs 24.2 m, p = 0.008) and PS ≥ 2 (8.8 vs 24.2 m, p = 0.012). In a multivariate model with PS, LDH, Ki-67 (55% cut-off) and ESR1 deletion, only elevated LDH (p = 0.047) kept its significant impact on OS whereas PS did not (p = 0.053). Among 13 NET G3 patients given cis/carboplatin and etoposide, the five with partial response had higher Ki-67 (mean 55.4 vs 35.9%, p = 0.082).
BRCA, ATM and other potential targets in NET G3/NEC
Among our 229 digestive HG-NEN, 10 (5.3%) of NEC and 6 (14%) of NET G3 harboured BRCA 1/2 mutations. Among the 8 cases with available normal tissue, none were germline mutations. We found no association between BRCA 1/2 mutation and RR to first-line cis/carboplatin and etoposide, neither for NEC (33 vs 38%, p = 0.84) nor for the 16 NEN combined (44 vs 37%, p = 0.67). BRCA 1/2 mutations had no significant impact on PFS or OS for NEN or NEC. We found ATM mutations among 7/188 (4%) and ATM deletions among 66/151 (44%) of NEC. We found no association between ATM deletions or -mutations and RR, PFS or OS. We found other targetable mutations infrequently among NEC; KRAS G12C mutations in 3%, RET mutations in 3% and HRAS mutation in 0.5%, but no ERBB2 amplification.
If adjustment for multiple testing, the only significant findings that withheld was longer survival for NET G3 compared to NEC and a significant longer survival for NEC with good performance status (PS 0–1).
Discussion
Treatment of metastatic digestive NEC patients is a clinical challenge as many patients progress immediately at the first evaluation after initiating first-line chemotherapy. For those with an initial response, treatment resistance develops rapidly with median PFS only 4–5 months and median OS 11–12 months [8, 10, 11]. There is a high unmet need for better predictive markers, more efficient treatment and, in general, more knowledge on this disease. Few previous studies have assessed treatment outcome according to genetic alterations for digestive NEC. Our study present novel findings regarding outcome after cis/carboplatin and etoposide for digestive HG-NEN patients according to genetic alterations, and for NEC these findings seems partly dependent on cell-type.
TP53 mutations or abnormal p53 protein expression is associated with platinum resistance for several cancers [18, 19]. TP53 mutations are frequent in NEC (51-89%) [15, 20,21,22]. In our study, TP53 mutation predicted inferior RR to platinum/etoposide in multivariate analyses across all NEC but did not correlate to OS. TP53 mutated SC-NEC, however, had a significantly better survival after platinum/etoposide. A recent study including pulmonary/digestive HG-NEN (N = 89) treated with cis/carboplatin and etoposide reported a numerical higher RR with inappropriate p53 (absence of or intense staining) [23]. A correlation between inferior survival and abnormal p53 expression/TP53 mutations have previously been reported for digestive HG-NEN [22, 24]. Most but not all TP53 mutations causes accumulation of p53 in the cell nuclei. This might lead to a relative discordance between protein p53 measurements and TP53 mutation frequency, and mutational analyses has been recommended for evaluation of clinical outcomes [25]. The prognostic value of TP53 mutations might depend on co-mutations and cancer type [26].
BRAF V600E mutations are frequent in colorectal NEC (28–47%), with a predominance in right-sided colon NEC [3, 27, 28]. BRAF mutations might be related to the particularly high frequency of treatment failure among colon NEC, where up to 65% have immediate progression on first-line treatment with cis/carboplatin and etoposide [6, 29]. In our study, no BRAF mutated NEC showed a response to first-line cis/carboplatin and etoposide, where 10/11 had colorectal origin. We found no OS differences dependent on BRAF status for NEC, a finding that could not be explained by possible differences in post first-line treatment. Our finding is in huge contrast to the substantial shorter survival found when BRAF mutation is present in metastatic colorectal adenocarcinoma supporting that colorectal NEC and adenocarcinoma are separate entities. Case studies have reported benefit of BRAF/MEK inhibition in BRAF mutated colorectal NEC [27, 30], and a BRAF/EGFR-inhibitor combination is approved for BRAF mutated CRC, not limited to adenocarcinomas. FDA recently approved a BRAF/MEK-inhibitor combination for BRAF mutated tumours, regardless of primary tumour site. Given our findings, BRAF V600E inhibition may be an attractive strategy for future trials on NEC.
RB1 deficiency is proposed as a marker predicting platinum effect for NEC, but results are conflicting [16, 31, 32]. RB1 mutations are infrequent in digestive NEC (14-25%), but RB1 deficiency, assessed by both genetic alterations and protein expression is more commonly reported (36-86%). The frequency of RB1 inactivation seems to differ according to primary site and is higher in SC compared to LC-NEC [3, 15, 33, 34]. In our data, no SC-NEC harbouring RB1 deletion experienced immediate progression on first-line cis/carboplatin and etoposide, as opposed to 38% of those without deletions. This is in line with previous published data on SCLC where RB1 mutations have been associated with improved responses, PFS and OS with platinum/etoposide [35]. We failed, however, to reproduce the impact on PFS and OS. While there are several mechanisms causing lack of proper pRb function, our data included only deletions and mutations and we may therefore underestimate the rate of pRb inactivation detected by immunohistochemical analysis. In a report on pancreatic NEC given platinum-treatment, RR was significantly higher for RB1 loss and/or KRAS mutation. Both RB1 loss and KRAS mutation predicted poor prognosis in univariate analyses, whereas only RB1 loss was prognostic in a multivariate model [16]. In a study on 54 HG-NEN,TP53 and/or BRAF mutations and immunohistochemical loss of Rb1- or p53 predicted shorter survival in univariate analyses [21].
MYC amplification is commonly described in both lung- and extra-pulmonary NEC, and proposed as a possible future therapy target [36]. Altered MYC has been linked to treatment resistance in several types of cancer [37]. A phase I study on MYC inhibition (OMO-103) reported effect among 22 patients with advanced solid tumours [38]. In our study, 7 (24%) of NET G3 and 74 (49%) of NEC had MYC amplification. Besides a non-significant trend towards decreased efficacy of cis/carboplatin and etoposide for MYC amplified NEC, we found no association between MYC status and treatment outcome nor survival.
BRCA mutations and ATM alterations. BRCA mutations are extremely rare in digestive adenocarcinomas (0.5-2%) [39], but its incidence among digestive HG-NEN has not been reported prior. Our findings of BRCA 1/2 mutations in 5.3% of NEC and 14.6% of NET G3 seem higher than for adenocarcinoma. ATM is mutated in ~5% of all cancers, but up to 10% of digestive adenocarcinoma [40]. Among 227 metastatic colorectal cancer, ATM mutations (15%) were correlated with superior OS [41]. We found ATM mutations in 4% and ATM deletions among 44% of our NEC cases. BRCA1/2 and ATM might be future new treatment targets in digestive NEC. BRCA 1/2 genes are critical in repair of double strand breaks through the homologous recombination repair (HRR) pathway. Alterations in these genes serve as predictive biomarkers to both platinum and PARP inhibitors [42]. Whether PARP inhibition has a role for treatment of NEC is, to our knowledge, not yet explored. ATM is also involved in HRR; however, its role as a predictive biomarker to DNA damage response agents is debated [43]. For both BRCA1/2 mutations and ATM alterations, we found no correlation to neither RR, PFS nor survival in platinum/etoposide treated cases. ATR protein inhibitors are under phase I/II investigation in NEC [44]. In a phase I study of ATR inhibitor elimusertib in solid tumours, all obtaining a partial response harboured ATM mutation or ATM protein loss [45].
NEC cell type
The clinical significance of digestive NEC cell-type is still unknown and both cell types are at present treated similarly. Some prior studies have reported a lower RR to platinum/etoposide in LC-NEC and an association with longer OS compared to SC-NEC [4, 46]. For SC-NEC we found a significantly higher RR compared to LC-NEC, a trend towards longer PFS but no impact on OS. When assessing genetic alterations according to cell-type for NEC, we found several correlations to outcome after cis/carboplatin and etoposide. For SC-NEC, RB1 deletion predicted disease control, ARID1A deletion immediate progression and TP53 mutation a significant better survival after platinum/etoposide. For LC-NEC, APC mutations predicted immediate progression and shorter PFS, whereas ARID1A deletion predicted disease control and longer PFS. Our results support that the classification of digestive NEC into large- and small cell is molecularly and clinically relevant. To our knowledge no prior study has reported on cell-specific outcome to treatment dependent on genetic alterations for NEC, and our findings should be validated in future trials.
NET G3
Surprisingly, NET G3 had a similar RR to platinum/etoposide compared to NEC, probably due to a high Ki-67 among NET G3 responders. This finding supports the updated NCCN guidelines suggesting consideration of platinum-compounds for NET G3 with high Ki-67 or aggressive behaviour [47]. For NET G3, we found no correlations between genetic alterations and response to chemotherapy or OS. ATRX mutation, ARID1A- and ERS1 deletions were associated with shorter PFS, but the sample size in these subgroups were very small. There are few published data on genomic aberrations and treatment outcomes for NET G3. In a recently presented study, a genomic signature of MEN1 mutation and DAXX-wild type correlated with longer PFS for pancreatic NET after capecitabine/ temozolomide treatment [48].
Clinical biomarkers
Poor PS is a well-known prognostic factor for digestive HG-NEN [6, 49]. In our data, LDH, CRP and PS were prognostic for NEC given cis/carboplatin and etoposide. In a multivariate model including only PS < 2, only elevated LDH was significantly associated with inferior survival. Elevated LDH has previously been shown to be prognostic for digestive NEC, and is one of the five variables included in the GI-NEC score [50]. When searching for novel prognostic molecular markers, still including the known traditional clinical markers seems important.
Strengths and limitations
We present prospectively collected data on one of the largest digestive HG-NEN cohorts described to date. However, sample size in several subgroups is still small. Our patient characteristics are similar to other studies published, indicating that the study cohort represents a real-life occurrence of digestive HG-NEN. Pathological expertise reassessed all cases according to the 2019 WHO classification. Presented genetic aberrations were limited to CNA and mutations, and a multi-omics approach would have provided a more in-depth information on aberrations that could influence treatment outcomes. Information on Rb-protein expression by immunohistochemistry could have added additional information but was not available due to the multi-centric approach. Multiple testing might increase the risk of false positive results. Although this is not normally done, we explored for this using the Benjamini- Hochberg procedure. Most of our results lost its significance after such an adjustment, likely due to our many small subgroups.
Summary
Correlations between genetic alterations and response/immediate progression to first-line cis/carboplatin and etoposide were frequent, but rarely affected PFS. In MVA, TP53 mutation was a significant predictor for inferior RR to cis/carboplatin for NEC. Except for a longer survival for TP53 mutated SC-NEC after platinum/etoposide, none of the investigated genetic alterations in our study was associated with a significant impact on OS in NEC. When separating NEC according to cell-type, several genetic alterations were correlated to efficacy indicating that the classification of digestive NEC into large cell and small cell is molecularly and clinically relevant. ATM alterations and BRCA mutations could be potential targets for novel therapeutic approaches. Several NET G3 genetic alterations were associated with PFS, however NET G3 cases were limited. When searching for novel prognostic molecular markers, still including the known traditional clinical markers seems important. The reason for the lack of substantial correlations between genetic alterations and OS is not obvious but could be due to the extreme aggressiveness of the disease with a very short PFS and OS. In future search for markers predicting treatment success, a multi-omics approach might be a way to better uncover the molecular mechanism behind the poor treatment outcome for digestive NEC.
Data availability
The data generated and analysed in this study are available from the corresponding author upon reasonable request.
References
WHO. WHO classification of digestive system tumours. 5th ed. Lyon: IARC; 2019.
Sorbye H, Baudin E, Perren A. The problem of high-grade gastroenteropancreatic neuroendocrine neoplasms: well-differentiated neuroendocrine tumors, neuroendocrine carcinomas, and beyond. Endocrinol Metab Clin North Am. 2018;47:683–98.
Venizelos A, Elvebakken H, Perren A, Nikolaienko O, Deng W, Lothe IMB, et al. The molecular characteristics of high-grade gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2021;29:1–14.
Elvebakken H, Perren A, Scoazec JY, Tang LH, Federspiel B, Klimstra DS, et al. A consensus-developed morphological re-evaluation of 196 high-grade gastroenteropancreatic neuroendocrine neoplasms and its clinical correlations. Neuroendocrinology. 2021;111:883–94.
Heetfeld M, Chougnet CN, Olsen IH, Rinke A, Borbath I, Crespo G, et al. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2015;22:657–64.
Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152–60.
Walter T, Tougeron D, Baudin E, Le Malicot K, Lecomte T, Malka D, et al. Poorly differentiated gastro-entero-pancreatic neuroendocrine carcinomas: are they really heterogeneous? Insights from the FFCD-GTE national cohort. Eur J Cancer. 2017;79:158–65.
Morizane C, Machida N, Honma Y, Okusaka T, Boku N, Kato K, et al. Effectiveness of etoposide and cisplatin vs irinotecan and cisplatin therapy for patients with advanced neuroendocrine carcinoma of the digestive system: the TOPIC-NEC phase 3 randomized clinical trial. JAMA Oncol. 2022;8:1447–55.
Strosberg JR, Coppola D, Klimstra DS, Phan AT, Kulke MH, Wiseman GA, et al. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas. 2010;39:799–800.
Eads JR, Halfdanarson TR, Asmis T, Bellizzi AM, Bergsland EK, Dasari A, et al. Expert Consensus Practice Recommendations of the North American Neuroendocrine Tumor Society for the management of high grade gastroenteropancreatic and gynecologic neuroendocrine neoplasms. Endocr Relat Cancer. 2023;30:e220206.
Sorbye H, Grande E, Pavel M, Tesselaar M, Fazio N, Reed NS, et al. European Neuroendocrine Tumor Society (ENETS) 2023 Guidance Paper for Digestive Neuroendocrine Carcinoma. J Neuroendocrinol. 2023;35:e13249.
de Mestier L, Lamarca A, Hernando J, Zandee W, Alonso-Gordoa T, Perrier M, et al. Treatment outcomes of advanced digestive well-differentiated grade 3 NETs. Endocr Relat cancer. 2021;28:549–61.
Malone ER, Oliva M, Sabatini PJB, Stockley TL, Siu LL. Molecular profiling for precision cancer therapies. Genome Med. 2020;12:8.
van Riet J, van de Werken HJG, Cuppen E, Eskens F, Tesselaar M, van Veenendaal LM, et al. The genomic landscape of 85 advanced neuroendocrine neoplasms reveals subtype-heterogeneity and potential therapeutic targets. Nat Commun. 2021;12:4612.
Wu H, Yu Z, Liu Y, Guo L, Teng L, Guo L, et al. Genomic characterization reveals distinct mutation landscapes and therapeutic implications in neuroendocrine carcinomas of the gastrointestinal tract. Cancer Commun. 2022;42:1367–86.
Hijioka S, Hosoda W, Matsuo K, Ueno M, Furukawa M, Yoshitomi H, et al. Rb loss and KRAS mutation are predictors of the response to platinum-based chemotherapy in pancreatic neuroendocrine neoplasm with grade 3: a Japanese multicenter pancreatic NEN-G3 study. Clin Cancer Res. 2017;23:4625–32.
Yates LR, Gerstung M, Knappskog S, Desmedt C, Gundem G, Van Loo P, et al. Subclonal diversification of primary breast cancer revealed by multiregion sequencing. Nat Med. 2015;21:751–9.
Reles A, Wen WH, Schmider A, Gee C, Runnebaum IB, Kilian U, et al. Correlation of p53 mutations with resistance to platinum-based chemotherapy and shortened survival in ovarian cancer1. Clin Cancer Res. 2001;7:2984–97.
Lin S, Li X, Lin M, Yue W. Meta-analysis of P53 expression and sensitivity to platinum-based chemotherapy in patients with non-small cell lung cancer. Medicine. 2021;100:e24194.
Konukiewitz B, Schlitter AM, Jesinghaus M, Pfister D, Steiger K, Segler A, et al. Somatostatin receptor expression related to TP53 and RB1 alterations in pancreatic and extrapancreatic neuroendocrine neoplasms with a Ki67-index above 20. Mod Pathol. 2017;30:587–98.
Busico A, Maisonneuve P, Prinzi N, Pusceddu S, Centonze G, Garzone G, et al. Gastroenteropancreatic high-grade neuroendocrine neoplasms (H-NENs): histology and molecular analysis, two sides of the same coin. Neuroendocrinology. 2019:Sep 27. https://doi.org/10.1159/000503722.
Joseph NPA, Le B, Moon F, Zhang L, Bergsland E. TP53 mutation portends a worse overall survival in patients with advanced grade 3 well-differentiated neuroendocrine tumors. Endocr Abstr. 2022;89:C40.
Lacombe C, De Rycke O, Couvelard A, Turpin A, Cazes A, Hentic O, et al. Biomarkers of Response to Etoposide-Platinum Chemotherapy in Patients with Grade 3 Neuroendocrine Neoplasms. Cancers. 2021;13:643.
Ali AS, Grönberg M, Federspiel B, Scoazec JY, Hjortland GO, Grønbæk H, et al. Expression of p53 protein in high-grade gastroenteropancreatic neuroendocrine carcinoma. PloS one. 2017;12:e0187667.
Soussi T, Béroud C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nat Rev Cancer. 2001;1:233–40.
Schaafsma E, Takacs EM, Kaur S, Cheng C, Kurokawa M. Predicting clinical outcomes of cancer patients with a p53 deficiency gene signature. Sci Rep. 2022;12:1317.
Capdevila J, Arques O, Hernandez Mora JR, Matito J, Caratu G, Mancuso FM, et al. Epigenetic EGFR gene repression confers sensitivity to therapeutic BRAFV600E blockade in colon neuroendocrine carcinomas. Clin cancer Res. 2020;26:902–9.
Dizdar L, Werner TA, Drusenheimer JC, Mohlendick B, Raba K, Boeck I, et al. BRAF(V600E) mutation: a promising target in colorectal neuroendocrine carcinoma. Int J Cancer. 2018;144:1379–90.
Elvebakken, Hjortland H, Garresori GO, Andresen H, Janssen EAM PA, Vintermyr OK, et al. Impact of KRAS and BRAF mutations on treatment efficacy and survival in high-grade gastroenteropancreatic neuroendocrine neoplasms. J Neuroendocrinol. 2023;35:e13256.
Klempner SJ, Gershenhorn B, Tran P, Lee TK, Erlander MG, Gowen K, et al. BRAFV600E mutations in high-grade colorectal neuroendocrine tumors may predict responsiveness to BRAF-MEK combination therapy. Cancer Discov. 2016;6:594–600.
Hadoux J, Kanaan C, Durand A, Hescot S, Hautefeuille V, Cadiot G, et al. Prognostic factors of metastatic neuroendocrine carcinoma under first-line treatment with platinum etoposide with a focus on NEC score and Rb expression: Results from the multicentre RBNEC study of the Groupe d’Etude des Tumeurs Endocrines (GTE) and the ENDOCAN-RENATEN network. Eur J Cancer. 2021;152:100–15.
Hadoux J, Walter T, Kanaan C, Hescot S, Hautefeuille V, Perrier M, et al. Second-line treatment and prognostic factors in neuroendocrine carcinoma: the RBNEC study. Endocr Relat Cancer. 2022;29:569–80.
Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surgical Pathol. 2012;36:173–84.
Takizawa N, Ohishi Y, Hirahashi M, Takahashi S, Nakamura K, Tanaka M, et al. Molecular characteristics of colorectal neuroendocrine carcinoma; similarities with adenocarcinoma rather than neuroendocrine tumor. Hum Pathol. 2015;46:1890–900.
Dowlati A, Lipka MB, McColl K, Dabir S, Behtaj M, Kresak A, et al. Clinical correlation of extensive-stage small-cell lung cancer genomics. Ann Oncol. 2016;27:642–7.
Frizziero M, Kilgour E, Simpson KL, Rothwell DG, Moore DA, Frese KK, et al. Expanding therapeutic opportunities for extrapulmonary neuroendocrine carcinoma. Clin Cancer Res. 2022;28:1999–2019.
Donati G, Amati B. MYC and therapy resistance in cancer: risks and opportunities. Mol Oncol. 2022;16:3828–54.
Garralda E, Moreno V, Alonso G, Corral E, Hernandez-Guerrero T, Ramon J, et al. Dose escalation study of OMO-103, a first in class Pan-MYC-Inhibitor in patients (pts) with advanced solid tumors. Eur J Cancer. 2022;174:S5–S6.
Zimmer K, Kocher F, Puccini A, Seeber A. Targeting BRCA and DNA damage repair genes in GI cancers: pathophysiology and clinical perspectives. Front Oncol. 2021;11:662055.
Tate JG, Bamford S, Jubb HC, Sondka Z, Beare DM, Bindal N, et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 2018;47:D941–D7.
Randon G, Fucà G, Rossini D, Raimondi A, Pagani F, Perrone F, et al. Prognostic impact of ATM mutations in patients with metastatic colorectal cancer. Sci Rep. 2019;9:2858.
Mylavarapu S, Das A, Roy M. Role of BRCA mutations in the modulation of response to platinum therapy. Front Oncol. 2018;8:16.
Choi M, Kipps T, Kurzrock R. ATM mutations in cancer: therapeutic implications. Mol Cancer Ther. 2016;15:1781–91.
Robinson MD, Livesey D, Hubner RA, Valle JW, McNamara MG. Future therapeutic strategies in the treatment of extrapulmonary neuroendocrine carcinoma: a review. Ther Adv Med Oncol. 2023;15:17588359231156870.
Yap TA, Tan DSP, Terbuch A, Caldwell R, Guo C, Goh BC, et al. First-in-human trial of the oral ataxia telangiectasia and RAD3-related (ATR) inhibitor BAY 1895344 in patients with advanced solid tumors. Cancer Discov. 2021;11:80–91.
Abdel‐Rahman O, Fazio N. Outcomes of small‐cell versus large‐cell gastroenteropancreatic neuroendocrine carcinomas: a population‐based study. J Neuroendocrinol. 2021;33:e12971.
Shah MH, Goldner WS, Benson AB, Bergsland E, Blaszkowsky LS, Brock P, et al. Neuroendocrine and adrenal tumors, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2021;19:839–68.
Lee P, Blais EM, Gong J, Osipov A, Moshayedi N, Thomassian S, et al. Genomic correlates of response to capecitabine and temozolomide (CAPTEM) in pancreatic neuroendocrine tumors. J Clin Oncol. 2022;40:4124.
Abdelmalak R, Lythgoe MP, Evans J, Flynn M, Waters J, Webb A, et al. Exploration of novel prognostic markers in grade 3 neuroendocrine neoplasia. Cancers (Basel). 2021;13:4232.
Lamarca A, Walter T, Pavel M, Borbath I, Freis P, Nuñez B. et al. Design and validation of the GI-NEC score to prognosticate overall survival in patients with high-grade gastrointestinal neuroendocrine carcinomas. J Natl Cancer Inst. 2017;109:djw277. https://doi.org/10.1093/jnci/djw277.
Acknowledgements
We would like to thank Randi Eikesdal (Clinical Cancer Research. Office, Haukeland University Hospital, Bergen, Norway) for data management and Dagfinn Ekse for technical assistance.
Funding
This work was supported by the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology (NTNU).
Author information
Authors and Affiliations
Contributions
Conceptualisation: HS and SK. Acquisition of data: HE, GOH, JS, HG, CK, EH, SD, LWV, HS. Analyses and data interpretation: HE, AV, TAM, SK. Pathological re-evaluation: AP, AC and IMBL. Writing original draft: HE, SK and HS. Supervision: SK and HS. All authors was involved in review and revision of the manuscript, and all approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
HG: Honoraria from Ipsen, Amgen, Pfizer, Bristol Meyer Squibb and Astra Zeneca, SK: Research support from AstraZeneca, Pfizer and Illumina and honoraria from AstraZeneca, Pfizer, Pierre- Fabre, Novartis, Sobi, Amgen, Sanofi Aventis and Roche, HS: Consultant/advisory board: Hutchison, Bayer, ITM, Advanced Accelerator. Lecture honoraria: Novartis, Ipsen, Bayer, SAM Nordic, Pierre Fabre. The other authors have no disclosures.
Ethics
The study was conducted according to the Declaration of Helsinki, and approved by ethics committees in Norway (REK vest 2012/940), Sweden (REC Uppsala Dnr 2012/285) and Denmark (Region Hovedstaden H-4-2012-108). All patients signed written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41416_2024_2773_MOESM2_ESM.docx
Supplementary Table 1 Treatment outcome to ciscarboplatin and etoposide according to genetic alteration stratified by NEC cell type
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elvebakken, H., Venizelos, A., Perren, A. et al. Treatment outcome according to genetic tumour alterations and clinical characteristics in digestive high-grade neuroendocrine neoplasms. Br J Cancer (2024). https://doi.org/10.1038/s41416-024-02773-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41416-024-02773-w
- Springer Nature Limited