Abstract
Background
Prostate cancer (PC) aetiology is unclear. PC risk was examined in relation to several factors in a large population-based prospective study.
Methods
Male participants were from Sax Institute’s 45 and Up Study (Australia) recruited between 2006 and 2009. Questionnaire and linked administrative health data from the Centre for Health Record Linkage and Services Australia were used to identify incident PC, healthcare utilisations, Prostate Specific Antigen (PSA) testing reimbursements and dispensing of metformin and benign prostatic hyperplasia (BPH) prescriptions. Multivariable Cox and Joint Cox regression analyses were used to examine associations by cancer spread, adjusting for various confounders.
Results
Of 107,706 eligible men, 4257 developed incident PC up to end 2013. Risk of PC diagnosis increased with: PC family history (versus no family history of cancer; HRadjusted = 1.36; 95% CI:1.21–1.52); father and brother(s) diagnosed with PC (versus cancer-free family history; HRadjusted = 2.20; 95% CI:1.61–2.99); severe lower-urinary-tract symptoms (versus mild; HRadjusted = 1.77; 95% CI:1.53–2.04) and vasectomy (versus none; HRadjusted = 1.08; 95% CI:1.00–1.16). PC risk decreased with dispensed prescriptions (versus none) for BPH (HRadjusted = 0.76; 95% CI:0.69–0.85) and metformin (HRadjusted = 0.57; 95% CI:0.48–0.68). Advanced PC risk increased with vasectomy (HRadjusted = 1.28; 95% CI:1.06–1.55) and being obese (versus normal weight; HRadjusted = 1.31; 95% CI:1.01–1.69).
Conclusion
Vasectomy and obesity are associated with an increased risk of advanced PC. The reduced risk of localised and advanced PC associated with BPH, and diabetes prescriptions warrants investigation.
Similar content being viewed by others
Introduction
Prostate cancer (PC) is the second most common cancer diagnosed in men worldwide and the most common cancer diagnosed in men from developed countries [1]. Australia has the highest incidence of PC internationally and, in 2021, an estimated 18,000 new PC cases will be diagnosed in Australia.
Despite its high incidence, there is limited evidence on risk factors for PC. Established risk factors include advancing age, African ancestry and family history of PC [2,3,4,5,6]. All these factors are non-modifiable, thus providing no basis for primary prevention of PC. While a diagnosis of cutaneous melanoma and obesity are associated with PC incidence, only the latter is potentially modifiable [7, 8]. There is emerging evidence that pharmaceuticals prescribed for the treatment of diabetes are related to the risk of a range of malignancies including PC [9,10,11,12,13,14,15]. Results from two large population-based prospective studies showed a reduced risk of incident PC in men taking pharmaceuticals for diabetes [16, 17]. It is suggested that the anti-tumour effects of prescriptions for diabetes may be due to their anti-proliferative effects.
Some urological factors are associated with PC risk including prescriptions for benign prostatic hyperplasia (BPH), lower-urinary-tract symptoms (LUTS) and vasectomy. These associations, especially with BPH prescriptions, vasectomy and LUTs may be at least partly attributable to increased medical surveillance [18,19,20,21]. The PSA test, the most commonly used biomarker in prostate cancer surveillance, was introduced in Australia in the late-1980s and the rise in its uptake corresponded with a rapid increase in PC incidence [22]. Thus, when seeking possible PC risk factors, the potential confounding effects of PSA testing on their associations with PC must be considered.
The current study prospectively examined potential risk factors for PC diagnosis in the 45 and Up Study, a large Australian population-based study of 267,153 male and female participants recruited in New South Wales (NSW). The study’s aim was to assess whether selected sociodemographic, behavioural and health-related factors were associated with a higher or lower risk of PC diagnosis, while accounting for possible confounding by PSA testing.
Methods
Study population
The Sax Institute’s 45 and Up Study is a large NSW population-based cohort study of male and female participants aged 45 years and above [23]. All participants were enrolled between 2006 and 2009. Participants were randomly sampled from Services Australia (formerly the Australian Government Department of Human Services) Medicare enrolment database: Services Australia is a publicly funded universal healthcare system that covers all citizens, permanent residents and some temporary residents and refugees. Those aged over 80 years and residents of regional areas were over-sampled by a factor of two. All participants completed a postal questionnaire at recruitment, which included information on sociodemographic factors, health behaviours and medical history and provided consent for linkage of their data to selected population health databases.
The 45 and Up Study was approved by the University of NSW Human Research Ethics Committee, while approval for this analysis of PC risk factors was given by the NSW Population and Health Services Research Ethics Committee (HREC/14/CIPHS/54). The use of Services Australia’s Medicare Benefits Schedule (MBS) and Pharmaceutical Benefits Scheme (PBS) data was approved by the Australian Department of Health’s Departmental Ethics Committee.
Record linkage
In 2017, the Centre for Health Record Linkage (CHeReL) linked records of all participants with selected administrative health records. These records were NSW Cancer Registry (NSWCR: January 1994–December 2013), Cause of Death Unit Record File (CODURF: February 2006–December 2015), Registry of Births, Deaths and Marriages records (RBDM: February 2006–December 2016) and NSW Admitted Patient Data Collection (APDC: July 2001–June 2016). All pathology laboratories, hospitals and radiotherapy and medical oncology departments are required by the Public Health Act 2010 to report all newly diagnosed cancers to the NSWCR. Information on the degree of spread at diagnosis is assigned within 4 months of PC diagnosis. However, if not all clinical information is received within this period of data acquisition, then these cases are classified as ‘Unknown’ spread [24, 25]. Probabilistic record linkage with these administrative health databases provided information on participants’ cancer diagnoses, procedures during admissions to public and private hospitals and death. In addition, the Sax Institute used a unique identifier to link study data deterministically with records of claims made to the Medicare Benefits Schedule (MBS: Sep 2005–Dec 2016) and Pharmaceutical Benefits Scheme (PBS: Sep 2005–Dec 2016) provided by Services Australia. This linkage provided information on PSA tests reimbursed by Medicare, General Practitioner (GP) consultations and relevant medications dispensed.
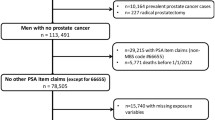
Exclusion criteria
-
(1)
PC diagnosis prior to recruitment to the 45 and Up Study (illustrated in Fig. 1). These were men with a record for PC diagnosis (ICD-10 C61) registered by the NSWCR, with APDC records listing C61 diagnosis codes or a radical prostatectomy recorded in Medicare Benefits Schedule (MBS) claims.
-
(2)
Participants with linkage errors in NSWCR, CODURF or RBDM records.
-
(3)
Department of Veterans’ Affairs (DVA) cardholders were excluded because a proportion of their medical and pharmaceutical benefits are funded by the Department of Veterans Affairs and these data were not available. DVA clients were identified via self-report or any mention of DVA coverage in hospital or emergency department records.
Exposure variables
The following PC risk factors of primary interest to this analysis were derived using self-reported information at recruitment:
family history of cancer (history of only PC, history of prostate and breast and/or ovarian cancers, history of other cancer(s) and no family history of cancer [reference]); family history of PC (father only, brother only, father and brother and no family history of PC [reference]); body mass index (BMI in kg/m2) based on height (m) and weight (kg) and categorised according to the World Health Organisation classification for normal (<24.9 kg/m2), overweight (25.0–29.9 kg/m2) or obese (≥30 kg/m2); [26] height divided into quartiles; weekly alcohol consumption categorised as non-drinkers with the remaining values divided into quartiles; physical activity from reported number of vigorous and non-vigorous physical activity sessions in a normal week consolidated and categorised as 0–3, 4–6, 7–10, 10–17, 18+ sessions per week; [27] LUTS measured using the modified International Prostate Symptom Score (m-IPSS) and categorised as having no, mild (0–5), moderate (6–11) and severe symptoms (12–21); [28] erectile dysfunction (ED) categorised as men who reported they can always or usually get and keep an erection firm enough for satisfactory sexual activity (No) and men who could not (Yes); smoking (Ever, Never) and vasectomy (Yes, No).
The following variables were obtained by linkage with PBS, MBS and APDC records up to 3 years before PC diagnosis or the censoring date: dispensing of medications to treat BPH (Anatomical Therapeutic Classification (ATC) code C02CA01, C02CA04, D11AX10 and G04C), to treat diabetes using metformin (PBS ATC code A10BA02) or non-metformin medications (PBS ATC code beginning with A10 but excluding A10BA02) with subsidised measurement of glycosylated haemoglobin (MBS 66551) or APDC code indicating diabetes contributed to need for a hospitalisation. Those with missing information for a single variable were included in the analysis as a “missing” category.
Outcome variable
PC cases were defined as men with a record for ICD-10 code C61 cancer registered with the NSWCR after enrolment in the 45 and Up Study and before the end of follow-up on 31 December 2013 (the last date for which data used in these analyses was available).
Potential confounders
Potential confounders available in the current cohort study based on previous publications were identified and referenced [2, 5, 6].
All participants’ and, separately, PC cases’ sociodemographic, lifestyle and behavioural characteristics, as self-reported at the time of recruitment, and relevant health-related factors obtained by linkage to administrative records are listed in Table 1. The place of residence classified according to the Accessibility Remoteness Index of Australia (ARIA+) was derived from the residential postcode. The following variables were obtained by linkage to MBS and APDC for which data were available from June 2004: Charlson Comorbidity Index (CCI) for non-cancer comorbidities was measured using a score derived from diagnosis codes in hospital admissions records up to 3-year period prior to PC diagnosis or date of censoring; uptake of PSA testing was based on MBS claims made from September 2005 until the date of PC diagnosis (or censoring date) for the MBS items for PSA screening (#66655) and monitoring (#66656 or #66659 or #66660), calculated as a rate per 5 years and grouped as having no record of PSA testing or monitoring (reference category), having a record for PSA monitoring but not PSA testing and having PSA testing at varying rates; frequency of MBS reimbursed visits to a general practitioner (GP) was calculated as a number of visits per 5 years and categorised into quartiles with 0–<12 visits as the reference category (Table 2).
Statistical analyses
Hazard ratios (HR) and 95% confidence intervals (CI) were estimated using Cox Proportional Hazards regression, with age as the underlying time variable, using SAS version 9 (SAS Institute Inc., Cary, NC, US). In these analyses, the endpoint was PC diagnosis or with censoring on the earlier of December 31, 2013, date death or the diagnosis date of cancer other than PC. Joint Cox Proportional Hazards regression, with age as the underlying time variable, was used to distinguish time to diagnosis based on the spread of disease defined as localised or advanced (regional and metastatic spread). Men with missing information to determine disease spread are classified as ‘unknown’ were excluded from the main Joint Cox regression analysis. The regression analyses were, at a minimum, adjusted for age, region of birth, health cover, income, qualifications, place of residence, marital status, Charlson comorbidity index, frequency of PSA testing, frequency of primary healthcare visits and fully adjusted for family history of cancer, BMI, smoking alcohol, physical activity, lower-urinary-tract symptoms, vasectomy, erectile dysfunction, medications for BPH and medications for diabetes (as listed in footnotes of each table).
Sensitivity analysis excluding the first year of follow-up for all participants after the date of enrolment in the 45 and Up Study was carried out to reduce the possible impact of reverse causation. Although our analysis examined incident PC in an era where PSA testing was widespread, we conducted a second sensitivity analysis to determine the impact of excluding a highly screened group. National Australian clinical practice guidelines for PSA testing and early management of test-detected PC, recommend men who have been informed of the benefits and harms of testing and who decide to undergo regular testing for prostate cancer, should be offered PSA testing every 2 years from age 50–69 [29]. Based on this, participants who had 3 or more PSA tests in the 5 years before the end of follow-up were defined as highly screened participants and were excluded.
Results
From the 267,153 participants in the 45 and Up Study, participants were excluded if they withdrew from the study after baseline (n = 268), were participants from the pilot study (n = 175), were aged below 45 years (n = 5) or were female (n = 142,929). Of the remaining 123,732 male participants, there were 107,706 eligible men for our analysis (Fig. 1). Of these, 4257 men were diagnosed with PC during the study period.
The median time between recruitment to study and PC diagnosis was 2.89 years. The median age of PC cases at recruitment was 65.9 years, 77.5% of men were born in Australia or New Zealand, 54.2% had private insurance cover, 50.9% were living in major cities and 81.8% reported being married or living with a partner (Table 1). Among PC cases, 8.2% reported having a family history of PC, had a median BMI of 26.6 kg/m2, 49.4% reported ever having smoked cigarettes, drank a median of 7 alcoholic drinks per week and did 10 h of median physical activity per week. Prior to PC diagnosis, there were 3.2% of PC cases identified as having a dispensed record for PBS subsidised prescription for metformin, and 9.9% of cases with prescription records for BPH treatment. Participants’ characteristics are summarised in Table 1. Sociodemographic and behavioural factors associated with increased risk of PC diagnosis were being born in Australia or New Zealand, and having private health cover, low income, no comorbidities, increased frequency of PSA testing and frequent visits to GP (Table 2).
Multivariable regression analyses in a fully adjusted model showed that the overall risk of PC diagnosis was highest in men with a family history of PC (HRadjusted = 1.36; 95% CI 1.21–1.52; Fig. 2), and in men with a brother and father diagnosed with PC (HRadjusted = 2.20; 95% CI 1.61–2.99). The risk of PC diagnosis was slightly lower in ever versus never smokers (HRadjusted = 0.91; 95% CI 0.85–0.97), while height, BMI, alcohol consumption and physical activity showed no association with PC diagnosis.
Analysis of urological factors showed an increased risk of PC diagnosis associated with increased severity of LUTS (Fig. 3; HRadjusted = 1.77; 95% CI: 1.53–2.05 for severe versus mild), and a weak association with vasectomy (HRadjusted = 1.08 95% CI: 1.00–1.16 for yes versus no); there was no association with previously reporting having emergency department records. The risk of PC diagnosis was lower in men with a record for PBS subsidised prescription for BPH treatment (HRadjusted = 0.76; 95% CI: 0.69–0.85 for yes versus no), and prescription for metformin (HRadjusted = 0.57; 95% CI: 0.48–0.68 for yes versus no) but no association with non-metformin medications for diabetes—Fig. 3.
Joint Cox regression analysis showed some evidence that the association between PC and vasectomy, and between PC and obesity, differed according to the stage of disease (p = 0.052 and p = 0.05, respectively) Specifically, men who had a vasectomy had a higher relative risk of advanced PC (HR = 1.28 95% CI: 1.06–1.55) than localised (HR = 1.04 95% CI: 0.95–1.14). Similarly, obese men (versus normal BMI) had a higher relative risk of advanced PC (HR = 1.31 95% CI: 1.01–1.69) than localised PC (HR = 0.98 95% CI: 0.87–1.12). There was no evidence of variation by stage of disease for all other exposures examined (Table 3).
The sensitivity analyses excluding all participants in the first year of follow-up (Supplementary Tables 1–3; Supplementary Figs. 1–3) and excluding frequent PSA testers (Supplementary Fig. 4), did not appreciably change the estimated HR (Supplementary Tables 4,5). Joint Cox regression analysis with the inclusion of PC cases with unknown status for stage showed no significant variation in HR estimates (p-value > 0.05) for all exposures examined (Supplementary Table 6).
Discussion
This study prospectively examined the evidence for several known or suspected causative or protective factors for PC. The risk of localised and advanced PC diagnosis was elevated in men with a family history of PC, multiple first-degree family members diagnosed with PC, severe LUTS, while the risk of advanced PC was increased in men who were obese or had a vasectomy. The overall PC risk was reduced in men with dispensed records for BPH medications or metformin. All analyses were adjusted for sociodemographic, lifestyle and health-related factors, as well as GP visits and frequency of PSA testing, prior to PC diagnosis.
Our findings for increased risk of being diagnosed with PC in men with a family history of PC, and a 2.5-fold risk in men with multiple first-degree family members with PC, is consistent with current evidence [3, 30,31,32]. PC is one of the most heritable malignancies with over 170 genetic variants identified to be associated with PC, in genome-wide association studies, which together account for 28.4% of the familial relative risk (compared to ~6% accounted for by rare genetic variants) [33, 34]. A meta-analysis of 33 epidemiological studies reported increased PC risk in men with a brother (RR = 3.14; 2.37–4.15) or father (RR = 2.35; 2.02–2.72) diagnosed with PC and this risk increased ~4-fold in men with 2 or more first-degree family members with PC (RR-4.39; 2.61–7.39). Given that family history is a combination of genetic, environmental and behavioural factors, the lower risk estimates observed in our study may in part be due to less variability in these factors due to the short follow-up period of 4–7 years since recruitment, as well as lack of information on the number of affected brothers, all of which may have biased results towards the null.
Our analysis of urological factors showed some evidence that for men who reported having a vasectomy, the relative risk of advanced PC was higher (HRadjusted = 1.28; 95% CI:1.06–1.55; p = 0.052) than the relative the relative risk of localised PC (HR = 1.04; 95% CI: 0.95–1.14). This finding is consistent with a 24-year follow-up of the Health Professionals Follow-Up Study, which reported no association between vasectomy and localised or low-grade disease, but an increased risk of high-grade (hazard ratio [HR], 1.22; 95% CI, 1.03–1.45) and advanced-stage prostate cancer (HR, 1.20; 95% CI, 1.03–1.40) [35]. A 15-year follow-up of participants from the European Prospective Investigation into Cancer and Nutrition (EPIC) study reported no association between vasectomy and overall or advanced PC; association by tumour grade was not reported [36]. A meta-analysis of 13 cohort studies showed weak evidence for a positive association between vasectomy and PC incidence (HR = 1.08; 95% CI 1.02, 1.14), but association by grade or stage of disease was not reported [37]. The biological mechanism underlying an association between vasectomy and PC risk is unclear.
The risk of localised and advanced PC was increased with increasing severity of LUTS but decreased in men with the prescription record(s) for BPH treatment. The role of LUTS in the etiologic development of PC and BPH share much in common and thus estimating the true association between LUTS and PC risk is complicated. LUTS is a self-reported measure, defined by increased frequency of urination, incomplete emptying of the bladder or waking during the night to void. It may be caused by BPH through direct obstruction of the bladder outlet or, may be associated with PC when the developing neoplasm exerts pressure on the bladder outlet or the urethra [38]. Results from two large cohorts of men who had all undergone PSA testing, the Prostate testing for cancer and Treatment study from UK (ProtecT) and Stockholm3 study from Sweden, reported no association between LUTS and PC risk [39, 40]. However the HUNT2 study from Norway reported an increased risk of localised but not advanced PC [41]. This uncertainty may partly be due to diagnostic bias such as increased PSA testing [21]. Despite controlling for frequency of PSA testing prior to PC diagnosis, a ~2-fold increased incidence of PC in men with severe LUTS was still evident in our study.
BPH is a non-malignant enlargement of the prostate that is clinically diagnosed. Severe BPH symptoms are commonly treated with minimally invasive surgery while mild to moderate symptoms are treated with medications that can shrink the prostate and ease urination. A systematic review of 8 trials showed that 5-alpha-reductase inhibitors (5-ARIs), a class of pharmaceutical prescriptions used in the treatment of BPH reduced the risk of PC diagnosis among men who regularly screened for PC. The Prostate Cancer Prevention Trial (PCPT) and the Reduction by Dutasteride of Prostate Cancer Event trial (REDUCE) which were specifically designed to examine the impact of 5-ARIs (finasteride and dutasteride) on PC risk, reported an overall 22.5–24.8% decreased risk of low-risk PC, but an increased incidence of high-risk disease [42,43,44]. Similar associations were reported in a 20-year follow-up of BPH cases [45]. We were unable to categorise PC cases by disease grade; however, our findings of reduced PC risk in localised and advanced PC suggest the association may be present in localised high risk as well as advanced cases. Further investigation in men with clinically significant PC may provide a better understanding of this association.
Metformin, commonly used as first-line therapy for diabetes which is the fastest growing chronic condition in Australia, may have a role in PC prevention due to its glucose-lowering and anti-proliferative properties [9, 11, 15, 17, 46, 47]. Diabetics have reduced PSA levels and given that biopsies are conducted in response to elevated PSA levels, this may delay PC diagnosis in diabetics and consequently lead to a diagnosis of more advanced disease However our results showed that the risk of localised as well as advanced PC diagnosis was reduced in men with prescription records for metformin. There is uncertainty in the evidence for an association between metformin and PC risk, and some of these inconsistencies may partly be due to the selection of study populations [11, 48]. The Finnish Randomised Study of Screening for Prostate Cancer showed that the risk of incident PC was lower in anti-diabetic drug users versus non-users (0.81; 0.61–0.95); however, sub-group analyses within diabetics, showed no association with PC incidence [17]. ‘Non-users’ of metformin, which is the reference group used in these studies, in a general population is a group that is generally free of diabetes, whereas ‘non-users’ in a diabetic population is a group that is diagnosed with diabetes but have chosen not to take medication. As such the effect in ‘users’ compared to ‘non-users’ in diabetics may be attenuated compared to that in the general population. Analyses restricted to diabetics are consistent with that reported by most studies which may have biased results towards null [11, 16, 48]. Various in vitro and clinical studies have associated elevated circulating insulin levels with tumour growth and increased cell proliferation. Metformin has the capacity to reduce tumour proliferation in a multitude of mechanisms that include reducing circulating insulin and glucose levels as well as regulating the expression of various cell signalling pathways involved in the regulation of insulin and glucose metabolism [49]. One small clinical trial of metformin in PC cases showed that metformin distributed to human prostate tissue, suggesting that metformin could exert its effects directly on tissue targets [50]. There are a few randomised clinical trials currently underway that will determine the potential chemoprevention properties of metformin for PC [51].
Obese men in our study had a higher relative risk of advanced PC diagnosis (HR = 1.54; 95% CI: 1.11–2.13) than localised PC (HR = 1.00; 95% CI: 0.84–1.20) (p = 0.05 for difference between HRs) and this is consistent with previous reports [7, 8]. We have previously reported higher PSA testing rates for obese men, but our recent study of this cohort reported lower biopsy rates but a higher likelihood of positive biopsy for obese men compared to men with a normal BMI [52, 53]. There is evidence that PSA levels are inversely associated with BMI, which may delay the diagnosis of PC [54]. Also, increased PC risk may be associated with obesity in men with high-grade disease. Men classified with localised disease in our study are a heterogeneous population with low to high-risk diseases diagnosed at an early stage. This heterogeneity may have attenuated the association and biased the hazards ratio towards the null [55]. Analysis by grade of disease was not possible.
The study sample comprises approximately 10% of the total population of NSW aged 45 and above and, consistent with most cohort studies, has a relatively modest response rate of 18% [56]. However, the associations quantified here are based on internal comparisons and previous methodological work reported that such comparisons are valid even though the cohort is not fully representative of the target population [56]. Research study participants as a group are healthier than the general population and may be more engaged in testing and screening behaviours than the general population, increasing the potential risk of diagnostic bias. Adjustments for PSA testing by previous studies have compared risk in the pre- and post-PSA testing eras, adjusted for baseline PSA levels, or adjusted for self-reported PSA testing from information collected at baseline [5, 35, 57]. Our ability to adjust for the rate of PSA testing, GP visits and other health comorbidities in the period prior to PC diagnosis (or censoring) for each participant through administrative record linkage, allowed us to minimise the risk of confounding related to differential levels of medical surveillance, is a strength. Joint Cox regression analysis was used to examine the association between each risk factor and the time to diagnosis for each stage of PC. This may have minimised the risk of error measurements in the time-dependent variables, which in this case is the spread of disease. Linkage with PBS data allowed us to derive information on dispensing of medications for diabetes and BPH, in the period prior to PC diagnosis (or censoring) for each participant. A limitation is that PBS data were only available since 2002 and therefore analysis for the duration of use was not possible. Furthermore, PBS data before 2012 used in this study, do not include data for medicines costing below the general beneficiary co-payment and so information for these medications for non-concessional patients may be missing. However, a validation study by Comino et al showed that the use of reimbursement records for diabetes prescriptions for 2002–2004 had a positive predictive value of ~85% for study participants who reported having diabetes in the baseline questionnaire, and this suggests that the effect of underestimation may be minimal [58]. Our classification of localised PC cases does not separate those with clinically significant disease from those with low risk or indolent cancers due to a lack of information on clinical pathology, and this is a limitation.
Since the early 1990s, PSA testing in Australia increased substantially with corresponding increases in the diagnosis of localised cancers and a fall in the rate of advanced cancers. Increased PSA testing has been documented in higher socio-economic status groups and in those with ‘healthier’ behaviours [53]. Therefore, adjusting for PSA testing behaviour is important in understanding the risk factors in localised (more likely to be screen-detected) and advanced cancers separately. Our findings show a range of established and emerging factors were associated with the risk of a diagnosis of localised and advanced PC, after adjusting for participants’ history of PSA testing. Further research is required to determine the association of these risk factors in the diagnosis of clinically significant PC.
Data availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. We obtained the data for the project from a third party, namely the Sax Institute, which is the data custodian for the 45 and Up Study. Data are available through application to the Sax Institute. Details are available at https://www.saxinstitute.org.au/our-work/45-up-study/ or through contacting 45andUp.research@saxinstitute.org.au.
References
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–53.
Giles GG, Severi G, English DR, McCredie MR, MacInnis R, Boyle P, et al. Early growth, adult body size and prostate cancer risk. Int J Cancer. 2003;103:241–5.
Hayes RB, Liff JM, Pottern LM, Greenberg RS, Schoenberg JB, Schwartz AG, et al. Prostate cancer risk in U.S. blacks and whites with a family history of cancer. Int J Cancer. 1995;60:361–4.
Hur J, Giovannucci E. Racial differences in prostate cancer: does timing of puberty play a role? Br J Cancer. 2020;123:349–54.
Perez-Cornago A, Key TJ, Allen NE, Fensom GK, Bradbury KE, Martin RM, et al. Prospective investigation of risk factors for prostate cancer in the UK Biobank cohort study. Br J Cancer. 2017;117:1562–71.
Nair-Shalliker V, Yap S, Nunez C, Egger S, Rodger J, Patel MI. et al. Adult body size, sexual history and adolescent sexual development, may predict risk of developing prostate cancer: Results from the New South Wales Lifestyle and Evaluation of Risk Study (CLEAR). Int J Cancer. 2017;140:565–74.
World Cancer Research Fund. Diet, Nutrition, Physical Activity and Cancer: a Global Perspective. https://www.wcrf.org/diet-activity-and-cancer/cancer-types/prostate-cancer/. Accessed May 2022.
Discacciati A, Orsini N, Andersson SO, Andrén O, Johansson JE, Wolk A. Body mass index in early and middle-late adulthood and risk of localised, advanced and fatal prostate cancer: a population-based prospective study. Br J Cancer. 2011;105:1061–8.
Feng T, Sun X, Howard LE, Vidal AC, Gaines AR, Moreira DM, et al. Metformin use and risk of prostate cancer: results from the REDUCE study. Cancer Prev Res. 2015;8:1055–60.
Feng, X, Song, M, Preston, MA, Ma, W, Hu, Y, Pernar, CH, et al. The association of diabetes with risk of prostate cancer defined by clinical and molecular features. Br J Cancer. 2020. https://doi.org/10.1038/s41416-020-0910-y.
Feng Z, Zhou X, Liu N, Wang J, Chen X, Xu X. Metformin use and prostate cancer risk: a meta-analysis of cohort studies. Medicine. 2019;98:e14955.
Ghiasi B, Sarokhani D, Najafi F, Motedayen M, Dehkordi AH. The relationship between prostate cancer and metformin consumption: a systematic review and meta-analysis study. Curr Pharm Des. 2019;25:1021–9.
Mayo Clinic Patient Care and Health Information. https://www.mayoclinic.org/diseases-conditions/benign-prostatic-hyperplasia/diagnosis-treatment/drc-20370093. Accessed April 2022.
Liu Q, Tong D, Liu G, Gao J, Wang LA, Xu J, et al. Metformin inhibits prostate cancer progression by targeting tumor-associated inflammatory infiltration. Clin Cancer Res. 2018;24:5622–34.
Margel D, Urbach D, Lipscombe LL, Bell CM, Kulkarni G, Austin PC, et al. Association between metformin use and risk of prostate cancer and its grade. J Natl Cancer Inst. 2013;105:1123–31.
Häggström C, Van Hemelrijck M, Zethelius B, Robinson D, Grundmark B, Holmberg L, et al. Prospective study of Type 2 diabetes mellitus, anti-diabetic drugs and risk of prostate cancer. Int J Cancer. 2017;140:611–7.
Haring A, Murtola TJ, Talala K, Taari K, Tammela TL, Auvinen A. Antidiabetic drug use and prostate cancer risk in the Finnish Randomized Study of Screening for Prostate Cancer. Scand J Urol. 2017;51:5–12.
Deng T, Lin X, Duan X, He Z, Zhao Z, Zeng G. Prostate cancer patients can benefit from 5-alpha-reductase inhibitor treatment: a meta-analysis. PeerJ. 2020;8:e9282.
Jacobs EJ, Anderson RL, Stevens VL, Newton CC, Gansler T, Gapstur, SM. Vasectomy and Prostate Cancer Incidence and Mortality in a Large US Cohort. J Clin Oncol. 2016. https://doi.org/10.1200/JCO.2015.66.2361.
Lin WY, Chang YH, Lin CL, Kao CH, Wu HC. Erectile dysfunction and the risk of prostate cancer. Oncotarget. 2017;8:52690–8.
Weight CJ, Kim SP, Jacobson DJ, McGree ME, Boorjian SA, Thompson RH, et al. The effect of benign lower urinary tract symptoms on subsequent prostate cancer testing and diagnosis. Eur Urol. 2013;63:1021–7.
Calopedos RJS, Bang A, Baade P, Yu XQ, Ruthven S, Patel MI, et al. Patterns of prostate-specific antigen testing by remoteness of residence and socio-economic status: an Australian population-based study. Aust J Rural Health. 2019;27:216–23.
Banks E, Redman S, Jorm L, Armstrong B, Bauman A, Beard J, et al. Cohort profile: the 45 and up study. Int J Epidemiol. 2008;37:941–7.
Cancer Institute of New South Wales, Caveat on the use of data items https://www.cancer.nsw.gov.au/getmedia/1d6bf5fd-eea3-48a4-b800-.
Cancer Institute of New South Wales, NSW Cancer Registry data dictionary, https://www.cherel.org.au/media/38825/nsw-cancer-registry-data-dictionary_may2015.pdf. Accessed May 2022.
WHO. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1-253
Banks E, Jorm L, Rogers K, Clements M, Bauman A. Screen-time, obesity, ageing and disability: findings from 91 266 participants in the 45 and Up Study. Public Health Nutr. 2011;14:34–43.
Latz I, Weber M, Korda R, Smith D, Clements M, Patel M, et al. Lower urinary tract symptoms in relation to region of birth in 95,393 men living in Australia: the 45 and Up Study. World J Urol. 2013;31:673–82.
Prostate Cancer Foundation of Australia and CancerCouncil Australia. Prostate Cancer Foundation of Australia and Cancer Council Australia PSA Testing Guidelines Expert Advisory Panel. Draft clinical practice guidelines for PSA testing and early management of test-detected prostate cancer. 2016. https://www.cancer.org.au/health-professionals/clinical-practice-guidelines/prostate-cancer. Accessed January 2022.
Ahn J, Moslehi R, Weinstein SJ, Snyder K, Virtamo J, Albanes D. Family history of prostate cancer and prostate cancer risk in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study. Int J Cancer. 2008;123:1154–9.
Bruner DW, Moore D, Parlanti A, Dorgan J, Engstrom P. Relative risk of prostate cancer for men with affected relatives: systematic review and meta-analysis. Int J Cancer. 2003;107:797–803.
Schuurman AG, Zeegers MP, Goldbohm RA, van den Brandt PA. A case-cohort study on prostate cancer risk in relation to family history of prostate cancer. Epidemiology. 1999;10:192–5.
Hjelmborg JB, Scheike T, Holst K, Skytthe A, Penney KL, Graff RE, et al. The heritability of prostate cancer in the Nordic Twin Study of Cancer. Cancer Epidemiol Biomark Prev. 2014;23:2303–10.
Mucci LA, Hjelmborg JB, Harris JR, Czene K, Havelick DJ, Scheike T, et al. Familial risk and heritability of cancer among twins in nordic countries. JAMA. 2016;315:68–76.
Siddiqui MM, Wilson KM, Epstein MM, Rider JR, Martin NE, Stampfer MJ, et al. Vasectomy and risk of aggressive prostate cancer: a 24-year follow-up study. J Clin Oncol. 2014;32:3033–8.
Smith K, Byrne, Castano JM, Chirlaque MD, Lilja H, Agudo A, et al. Vasectomy and prostate cancer risk in the european prospective investigation into cancer and nutrition (EPIC). J Clin Oncol. 2017;35:1297–303.
Bhindi B, Wallis CJD, Nayan M, Farrell AM, Trost LW, Hamilton RJ, et al. The association between vasectomy and prostate cancer: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:1273–86.
Abrams P, Chapple C, Khoury S, Roehrborn C, de la Rosette J. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol. 2009;181:1779–87.
Chandra Engel J, Palsdottir T, Aly M, Egevad L, Grönberg H, Eklund M, et al. Lower urinary tract symptoms (LUTS) are not associated with an increased risk of prostate cancer in men 50-69 years with PSA ≥3 ng/ml. Scand J Urol. 2020;54:1–6.
Collin SM, Metcalfe C, Donovan J, Lane JA, Davis M, Neal D, et al. Associations of lower urinary tract symptoms with prostate-specific antigen levels, and screen-detected localized and advanced prostate cancer: a case-control study nested within the UK population-based ProtecT (Prostate testing for cancer and Treatment) study. BJU Int. 2008;102:1400–6.
Martin RM, Vatten L, Gunnell D, Romundstad P, Nilsen TI. Lower urinary tract symptoms and risk of prostate cancer: the HUNT 2 Cohort, Norway. Int J Cancer. 2008;123:1924–8.
Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192–202.
Thompson IM, Pauler Ankerst D, Chi C, Goodman PJ, Tangen CM, Lippman SM, et al. Prediction of prostate cancer for patients receiving finasteride: results from the Prostate Cancer Prevention Trial. J Clin Oncol. 2007;25:3076–81.
Unger JM, Till C, Thompson IM, Tangen CM, Goodman PJ, Wright JD, et al. Long-term Consequences of Finasteride vs Placebo in the Prostate Cancer Prevention Trial. Journal of the National Cancer Institute. 2016;108:djw168:1-7.
Van Rompay MI, Curtis Nickel J, Ranganathan G, Kantoff PW, Solomon K, et al. Impact of 5α-reductase inhibitor and α-blocker therapy for benign prostatic hyperplasia on prostate cancer incidence and mortality. BJU Int. 2019;123:511–8.
Bansal D, Bhansali A, Kapil G, Undela K, Tiwari P. Type 2 diabetes and risk of prostate cancer: a meta-analysis of observational studies. Prostate Cancer Prostatic Dis. 2013;16:151–158, s151.
Preston MA, Riis AH, Ehrenstein V, Breau RH, Batista JL, Olumi AF, et al. Metformin use and prostate cancer risk. Eur Urol. 2014;66:1012–20.
He K, Hu H, Ye S, Wang H, Cui R, Yi L. The effect of metformin therapy on incidence and prognosis in prostate cancer: a systematic review and meta-analysis. Sci Rep. 2019;9:2218.
Gallagher EJ, LeRoith D. Diabetes, cancer, and metformin: connections of metabolism and cell proliferation. Ann N Y Acad Sci. 2011;1243:54–68.
Nguyen MM, Martinez JA, Hsu CH, Sokoloff M, Krouse RS, Gibson BA, et al. Bioactivity and prostate tissue distribution of metformin in a preprostatectomy prostate cancer cohort. Eur J Cancer Prev. 2018;27:557–62.
U.S. National Library of Medicine, Database of privately and publicly funded clinical studies https://clinicaltrials.gov/ct2/results?recrs=&cond=Prostate+Cancer&term=metformin&cntry=&state=&city=&dist=. Accessed May 2022.
Chiam K, Bang A, Patel MI, Nair-Shalliker V, O’Connell DL, Smith DP. Characteristics associated with the use of diagnostic prostate biopsy and biopsy outcomes in Australian men. Cancer Epidemiol Biomark Prev. 2021;30:1735–43.
Nair-Shalliker V, Bang A, Weber M, Goldsbury DE, Caruana M, Emery J, et al. Factors associated with prostate specific antigen testing in Australians: analysis of the New South Wales 45 and Up Study. Sci Rep. 2018;8:4261.
Bonn SE, Sjölander A, Tillander A, Wiklund F, Grönberg H, Bälter K. Body mass index in relation to serum prostate-specific antigen levels and prostate cancer risk. Int J Cancer. 2016;139:50–57.
Hu MB, Liu SH, Jiang HW, Bai PD, Ding Q. Obesity affects the biopsy-mediated detection of prostate cancer, particularly high-grade prostate cancer: a dose-response meta-analysis of 29,464 patients. PLoS ONE. 2014;9:e106677.
Mealing NM, Banks E, Jorm LR, Steel DG, Clements MS, Rogers KD. Investigation of relative risk estimates from studies of the same population with contrasting response rates and designs. BMC Med Res Methodol. 2010;10:26.
Randazzo M, Beatrice J, Huber A, Grobholz R, Manka L, Wyler SF, et al. Influence of metformin use on PSA values, free-to-total PSA, prostate cancer incidence and grade and overall survival in a prospective screening trial (ERSPC Aarau). World J Urol. 2015;33:1189–96.
Comino EJ, Tran DT, Haas M, Flack J, Jalaludin B, Jorm L, et al. Validating self-report of diabetes use by participants in the 45 and Up Study: a record linkage study. BMC Health Serv Res. 2013;13:481.
Acknowledgements
This research was completed using data collected through the 45 and Up Study (www.saxinstitute.org.au). The 45 and Up Study is managed by the Sax Institute in collaboration with major partner Cancer Council NSW; and partners: the Heart Foundation; NSW Ministry of Health; NSW Department of Communities and Justice; Australian Red Cross Lifeblood. We thank thousands of people participating in the 45 and Up Study and, the Centre for Health Record Linkage for the record linkage and Services Australia, the NSW Ministry of Health and Cancer Institute NSW for the use of their data.
Funding
DPS was supported by a grant from Cancer Institute NSW (#15/CDF/1-10). EB is supported by The National Health and Medical Research Council of Australia (ref1136128), which is independent of this publication. Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
VNS, SE, DO’C, BKA and DPS were involved in the concept development phase and the development of the analysis plan. AB undertook all data analyses and prepared tables. SE, XQY and DO’C had oversight of all statistical analyses. VNS prepared the first draft of the manuscript and updated all revisions. All authors have read the manuscript and provided feedback.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Individuals gave written informed consent to take part in the study, including consent for follow-up through repeated data collection and linkage of their data to population health databases. Ethical approval for the conduct of the 45 and Up Study was provided by the University of New South Wales Human Research Ethics Committee. Ethical approval for the present study was provided by the NSW Population & Health Services Research Ethics Committee (14/CIPHS/54).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nair-Shalliker, V., Bang, A., Egger, S. et al. Family history, obesity, urological factors and diabetic medications and their associations with risk of prostate cancer diagnosis in a large prospective study. Br J Cancer 127, 735–746 (2022). https://doi.org/10.1038/s41416-022-01827-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-01827-1
- Springer Nature Limited
This article is cited by
-
Does metformin really reduce prostate cancer risk: an up-to-date comprehensive genome-wide analysis
Diabetology & Metabolic Syndrome (2024)
-
Effect of metformin on incidence, recurrence, and mortality in prostate cancer patients: integrating evidence from real-world studies
Prostate Cancer and Prostatic Diseases (2024)