Abstract
Background Rates of oropharyngeal (OP) cancer are increasing and mortality is related to stage at diagnosis. Early diagnosis is vital to improving patient outcomes.
Aim To describe current general practice pathways and time intervals in OP cancer and: a) compare to current National Institute for Health and Care Excellence guidance to refer from general practitioners (GPs) to general medical dentists (dentists); and b) referral pathways for pharyngeal cancers.
Design and setting A ten-year retrospective study of patients diagnosed with OP cancer in one suburban general practice in England using GP notes, including secondary care correspondence.
Results There were 12 cases of OP cancer; six oral and six pharyngeal. There were marked differences in referral pathways and time intervals for people with visible, or palpable, oral cancers and those with non-visible, or impalpable, pharyngeal cancers. No one had GP to dentist referral. General practice 'safety-netting' or follow-up was not commonly recorded.
Conclusion GPs are pivotal in diagnosing symptomatic OP cancers. General practice and dental teams encountering symptoms of uncertain aetiology (for example, pharyngitis) should offer safety-netting to shorten patient intervals to re-attendance. Pathways for oral cancer referral were usually clear and linear. Pathways for pharyngeal cancer were usually complex, with much longer time intervals in primary and secondary care, and would benefit from a single national referral pathway to ENT.
Key points
-
Symptomatic oropharyngeal cancers in this study were all referred by GPs.
-
Oral cancers usually had much shorter primary care and total diagnostic intervals than pharyngeal cancers; the cancers should be decoupled in studies and management.
-
Preventing pharyngeal cancer referrals to gastroscopy, and instead directing to ear, nose and throat (ENT) or combined oral and maxillofacial surgery and ENT clinic, would shorten secondary care and diagnostic intervals for patients.
Similar content being viewed by others
Introduction
Head and neck cancer is the eighth most common cancer in the UK; rates have increased by 20% in the last decade,1 including increases in both oral and pharyngeal (OP) cancers. Despite increasing understanding of the scientific underpinning and risk factors for disease development,2,3,4 the death toll continues to rise, claiming the lives of about 4,000 people in the UK each year.1 At the time of histological diagnosis, it is common for OP cancer to have reached stage III or IV.5 Disease outcome is related to stage at diagnosis and so to length of diagnostic delay. There have been some criticisms of National Institute for Health and Care Excellence (NICE) guidance6 in relation to referral criteria, most recently updated in 2017, as it advocates for cross-referral between general practitioners (GPs) and dentists for lip lumps, oral cavity lumps, erythroplakia, leukoplakia or erythroleukoplakia.7,8 While there is opportunity for collaboration across primary care, variability in access and affordability of primary dental services (dentists) for many represents major barriers for some patients. There is a lack of auditable referral pathway and safety-netting processes, which limits this guidance and may introduce unnecessary diagnostic delay. On the other hand, dentists are experts at appraising oral lesions and so reducing inappropriate secondary care referrals.
Diagnosis of OP cancer is often challenging; patients may not progress sequentially through checkpoints, with potential for delay, due to:
-
Cancer factors; for example, non-specific symptoms and non-visibility
-
Practitioner factors; for example, knowledge, experience and referral quality
-
System factors; for example, waiting times and referral processes
-
Patient factors; for example, concern about symptoms, confidence, and early access to both dental and general practice services.
This case review describes the diagnostic journey in patients diagnosed with OP cancers, as captured from a GP perspective, with the aim of identifying key areas of diagnostic delay, with recommendations to shorten diagnostic intervals.
Methods
We performed a ten-year retrospective case note review of patients diagnosed with OP cancer at a large general practice in North West England (patient population 12,300). Health Research Authority approved - study number: IRAS project ID 262938. Using the search terms 'oral cancer', 'mouth cancer', 'lip cancer', 'tongue cancer', 'oropharyngeal cancer' and 'tonsillar cancer', we were able to retrieve the records of patients with a diagnosis of OP cancer from 1 January 2009 to 1 January 2019 (inclusive).
The medical records for each patient were reviewed by JW. Anonymised data were inputted to a data collection form for each participant (Appendix 1) and re-validated by JW a week after initial collection, with time intervals checked using an online date calculator and shared with co-researcher CG-C.
We used the Aarhus checklist,9 an internationally recognised framework in assessment of research quality in early cancer diagnosis research, to develop a data collection form with pre-defined checkpoints and times in the patient journey, to assess progression through primary care.
Cancer-specific details were site of primary lesion and histological subtype.
For each consultation between clinician and patient, data recorded were:
-
Medical history, physical examination, working diagnosis, investigations, treatment and follow-up arrangements
-
If follow-up arranged, the time interval between attendance and re-attendance was recorded
-
Referrals made were classified as: 'routine', 'urgent' or 'cancer' referral. In NHS England, cancer referrals require a two-week wait maximum to secondary care clinic visit from referral.
We recorded each primary care patient journey with:
-
Total number of primary care consultations before achievement of definitive diagnosis
-
Number of GP to dentist referrals
-
Intra-practice referrals between general practice team members.
A summary form for each patient, with a form for each consultation and secondary care outcomes from GP letters, was created to explore pathways in secondary care to diagnosis and patient movements between primary and secondary care.
The definition of OP cancer within cancer registries varies in published research and clinical guidelines.10 In this study, oral cavity cancer originated from the inner lip, gums, floor of mouth, palate and the anterior two-thirds of the tongue. Pharyngeal cancers originated from nasopharynx, oropharynx and hypopharynx. As we found marked differences in pathways between the two groups, we describe the patients in terms of:
-
Oral (visible) cancer, which in this series includes one palpable cancer
-
Pharyngeal (non-visible) cancer.
Results are discussed generally without specific patient discussion to increase anonymity. Patient cancer journeys were assigned a case number.
Results
Twelve OP cancer diagnoses were made during the study period, with diagnoses in the same individual at different time points in one individual (cases 1 and 6). Case 1 was a lip cancer, but re-presented several years after discharge from services as a new case (case 6), with palpable metastatic submental recurrence, within the study timeframe. Case 6 is not strictly oral cancer; it is a secondary from case 1, but is included in oral cancer pathways to distinguish from pharyngeal cancer cases. Primary care results are summarised in Table 1. Case 2 re-presented with recurrence at the previous primary cancer site, years after discharge from cancer services, via self-referral to their original secondary care cancer team. The initial cancer occurred before 2009, so is not included. One case had a lung cancer diagnosis at OP cancer diagnosis. Apart from smoking, two cases had alcoholism and one intravenous drug misuse, acting as risk factors and complicating presentations and care. One case had severe dementia, limiting therapy, and one chronic obstructive pulmonary disease. All cases were histological oral squamous cell carcinomas.
Six of the 12 cases (cases 1-6) were visible or palpable oral cavity presentations with subsites: lip (n = 1), buccal surface (n = 1), palate (n = 1), tongue (n = 2) and palpable submental lymph nodes (n = 1).
Six cancers were pharyngeal; not visible, nor palpable (cases 7-12) with subsites: supraglottic pyriform fossa (n = 4), lingual tonsil base (n = 1) and posterior pharyngeal wall (n = 1).
Oral cancers (cases 1-6) were different to pharyngeal cancers (cases 7-12) in presentation and pathways, so are reported separately. All cases were referred by GPs except case 2 (none by dentists) and all were symptomatic. There were no GP to dentist referrals and no intra-practice healthcare professional referrals for diagnosis; however, there were some for other care; for example, stop smoking services.
Primary care interval and pathways
Initial consultation was with a GP in ten out of 12 cases. One case consulted with a practice nurse initially and case 2 self-referred to secondary care.
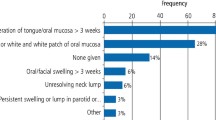
Oral cancer symptoms at general practice presentation were one each of: persistent lip ulceration, buccal leukoplakia, palatal erythroplakia and sensitivity, glossal leukoplakia and glossal ulceration. Case 6 was palpable submental lymphadenopathy as a new presentation from case 1.
Pharyngeal cancer symptoms at general practice presentation were: unexplained pharyngitis (n = 3), weight loss (n = 2), odynophagia (pain on swallowing) (n = 2), anorexia (n = 1) and cervical dysphagia (dysphagia above the suprasternal notch) (n = 1). Later symptoms, but before definitive diagnosis, included: mild dysphagia (food 'sticking') (n = 2), pharyngitis (n = 1), palpable neck nodes (n = 2), unilateral otalgia (n = 2) and anxiety (n = 1).
Oral cancers
As case 2 self-referred to secondary care, there were five cases with oral cancer primary care intervals.
There was clear GP record-keeping for symptoms and signs, and no investigations arranged. Recording of working diagnoses described presenting symptoms, not likely aetiology or possibility of cancer, even when sending a cancer referral in the consultation. One case was prescribed aciclovir. Only one case was offered follow-up or advice on when to return (safety-netting). Four cases were seen once and immediate referrals made - three referred on a cancer form and case 6 urgently. The maximum wait to sending the referral was three days. Primary care intervals for cases 3, 4, 5 and 6 ranged from 1-3 days.
Case 1 was complex, with six consultations before definitive referral, but was referred on first consultation routinely (initial primary care interval: six days) to oral and maxillofacial surgery (OMFS) outpatient clinic, with an 81-day wait to biopsy. Unfortunately, benign biopsy histology resulted in patient discharge and a subsequent patient interval of 263 days before re-presenting for second routine referral - as a persistent lesion at non-healing biopsy site. There were unrelated interval consultations, a missed second specialist appointment due to inpatient stay and final cancer referral by GP after recurrent, non-related, post-discharge home visit reviews. Apart from this nonlinear presentation (case 1 presented years later with palpable lymphadenopathy as case 6, illustrating how late diagnosis creates poorer outcomes), other oral cancer primary care intervals were much shorter and linear, suggesting successful pathways. As case 1 was referred on first general practice presentation and had histology been cancer-positive, it too would have been linear. However, sending case 1 as routine, rather than cancer referral, increased the primary care interval of six days to first diagnostic interval to benign biopsy of 81 days. In the end, the diagnostic interval to oral cancer diagnosis was 429 days.
Pharyngeal cancers
Pharyngeal cancer pathways tended to be more complex with interplay between secondary care, primary care and patient intervals, resulting in much longer diagnostic times (Table 1). Cases required a range of 2-7 GP consultations before secondary care referral. All pharyngeal cancers were referred as possible cancer and history clearly recorded. Examination was recorded in five of six cases and half had blood tests initially, resulting in one new diabetes diagnosis.
Diagnosis was recorded as a symptom: pharyngitis (n = 2), sore throat (n = 1), dysphagia (n = 1) and no record (n = 2), rather than suggesting likely aetiology, possible cancer cause or 'unknown aetiology'. Treatments offered were: amoxicillin (n = 2), Difflam spray (n = 2) and Fortisip (n = 1).
Once referral was decided, GPs sent the referral within two days. No cases were initially offered GP follow-up or advice on when to re-attend. One was referred to stop smoking services. This lack of recorded safety-netting possibly led to recurrent delays in re-attendance (patient intervals) as patients re-evaluated their symptoms before re-attending, often months later. As these primary care intervals are complex, they are briefly described below.
Case 7 has a primary care interval of 131 days, as the patient re-attended general practice with intermittent pharyngitis, with long patient intervals before GP referral to ear, nose and throat (ENT). A benign diagnosis was made, normal barium swallow undertaken and proton pump inhibitors given with follow-up stated. However, case 7 was lost to outpatient clinic (no further letters) and re-attended general practice after months, with other symptoms, and was re-referred to ENT. The resulting total diagnostic interval was 390 days to pathological diagnosis.
Case 8 had two GP consultations before gastroscopy cancer referral, with initial primary care interval of 24 days. Unable to gastroscope, case 8 had normal barium swallow and speech and language therapy (SALT) routine referral. One interval GP consultation occurred with pharyngeal cancer symptoms before SALT attended and contacted the GP to undertake same-day ENT cancer referral. Total primary care interval became 115 days with total diagnostic interval of 242 days.
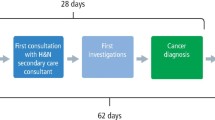
Case 9 attended twice in one week and was referred for cancer gastroscopy with one-day delay to referral being sent. The primary care interval was six days. The gastroenterology specialist immediately referred as a cancer to ENT, with a total diagnostic interval of 28 days.
Case 10 had comorbidities with intervening inpatient episodes and was referred on third general practice presentation for gastroscopy on a cancer referral, with 64-day primary care interval. Unable to gastroscope, case 10 was cross-referred as cancer to ENT. Initial biopsies were inconclusive, requiring repeat biopsy, and gave a total diagnostic interval of 124 days. Initial referral to ENT and first biopsy concluding cancer-positive would have shortened the diagnostic interval.
Case 11 was seen five times by GPs, with long intervening patient intervals before cancer referral to respiratory clinic and a primary care interval of 143 days. The respiratory specialist cross-referred as cancer to ENT, with total diagnostic interval of 159 days.
Case 12 was referred on first consultation for cancer gastroscopy, with primary care interval of 0 days (same day). Gastroscopy was normal, as was barium swallow, leading to outpatient clinic follow-up with concomitant medication and tests in general practice. There were seven GP consultations with follow-up offered within the secondary care interval, as the GP attempted to improve the patient symptomatology. Ultimately, the GP asked for early review and on presentation was cross-referred by gastroenterology to ENT. Total primary care interval to second definitive referral was 153 days. The diagnostic interval was 187 days. Initial referral to ENT by GP would have reduced the diagnostic interval.
The average interval between definitive diagnosis in secondary care and patients attending their multidisciplinary cancer team meetings was eight days for both oral cavity and pharyngeal cancers.
Discussion
We could not find another general practice case series in the UK on pathways and primary care intervals to OP cancer diagnoses. Patient case note review, rather than patient interview or questionnaire, prevented recollection errors. In contrast to previous works, in which a large proportion of diagnostic delay is attributed to the patient, our study suggests that patients are driven by symptom impact, seldom missing appointments despite complex comorbidity and engaged appropriately with treatments and investigations. When patients are not referred by GPs, follow-up and safety-netting symptoms should be recorded to help track symptoms; there may be a role for artificial intelligence in supporting this.
NICE guidelines for suspected oral cancer were updated in 20156 and recommend that 'GPs consider an urgent referral (for an appointment within two weeks) for assessment for possible oral cancer by a dentist in people who have either: a lump on the lip or in the oral cavity or a red or red and white patch in the oral cavity consistent with erythroplakia or erythroleukoplakia'.
Within our study, there were no GP to dentist referrals. Dentists are experienced in identification of benign lesions and may be an under-used resource for GPs. Two cases of oral cancer would have met NICE criteria for referral to dentists (erythroplakia and leukoplakia) but both were referred as possible cancers to secondary care. There is no auditable robust pathway for GP to dentist referrals; not everyone has access to a dentist or can afford private or NHS dentistry, so it may create delay. These areas need improvement if GP-dentist intercommunication is to work in the patient's favour and possibly reduce referrals to secondary care for benign conditions.
We recommend splitting OP cancers into oral cavity and pharyngeal, to improve scrutiny of primary and total diagnostic intervals. It is only after the GP or dental decision to refer, filling in the local cancer pro forma, that symptoms of pharyngeal cancer are described. NICE guidance should include odynophagia and cervical dysphagia symptoms. Scottish cancer guidelines include odynophagia,11 as does the Cancer Research UK website on 'site-specific cancers' and the oral cancer toolkit. The latter recommends against GP to dentist referrals12 at present. We have listed difficult symptoms which may suggest pharyngeal cancer (Table 2).
Secondary care referrals for oral cancer were dealt with by OMFS, which diagnosed all cases. We recommend possible pharyngeal cancers be referred to ENT first, or OMFS with dedicated ENT availability, as a one-stop shop (this does occur in many units). In this study, cases were referred by GPs, and between secondary care specialists, to gastroenterology, respiratory, SALT and ENT, sometimes repeatedly. One pharyngeal cancer was initially referred to ENT, but all cases were ultimately diagnosed by ENT. High dysphagia is a diagnostic problem for GPs and dentists, many thinking of upper oesophageal cancer and gastroscopy referral - with delay to diagnosis. ENT, with flexible nasopharyngo-laryngoscopy or transnasal-pharyngo-oesophagoscopy at first attendance, provides more accurate, less invasive initial diagnostic testing compared to gastroscopy, which was attempted in over half of our pharyngeal cancer cases, and would improve outcomes. Minimal sedation is required, the patient can converse and full post-nasal space, larynx and oropharynx can be visualised.
Conclusion
Important but uncommon, this study is limited by small OP cancer numbers, but we are confident that general practice and dental clinical teams will recognise similar pathways their patients have undertaken. Dentists triage benign and malignant OP cancers and we urge dentists, as well as GPs, to publish information on primary care and total diagnostic intervals to improve patient outcomes and help reach the faster diagnostic standards set for cancer diagnosis. The main imperative from this study for GPs was to offer safety-netting to reduce patient intervals, and for pharyngeal cancer to have a dedicated pathway in national guidance for referral and diagnosis.
References
Cancer research UK. Head and Neck Cancer statistics. Available at https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/head-and-neck-cancers (accessed June 2020).
Hashibe M, Brennan P, Chuang S-C et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev 2009; 18: 541-550.
Conway D I, Brenner D R, McMahon A D et al. Estimating and explaining the effect of education and income on head and neck cancer risk: INHANCE consortium pooled analysis of 31 case-control studies from 27 countries. Int J Cancer 2015; 136: 1125-1139.
Edefonti V, Hashibe M, Ambrogi F et al. Nutrient-based dietary patterns and the risk of head and neck cancer: a pooled analysis in the International Head and Neck Cancer Epidemiology consortium. Ann Oncol 2012; 23: 1869-1880.
Stathopoulos P, Smith W P. Analysis of survival rates following primary surgery of 178 consecutive patients with oral cancer in a large district general hospital. J Maxillofac Oral Surg 2017; 16: 158-163.
NICE. Suspected cancer: recognition and referral NICE guideline [NG12]. 2015. Available online at https://www.nice.org.uk/guidance/ng12 (accessed June 2020).
Grimes D, Patel J, Avery C. New NICE referral guidance for oral cancer: does it risk delay in diagnosis? Br J Oral Maxillofac Surg 2017; 55: 404-406.
Grafton-Clarke C, Chen K W, Wilcock J. Diagnosis and referral delays in primary care for oral squamous cell cancer: a systematic review. Br J Gen Pract 2019; 69: 112-126.
Weller D, Vedsted P, Rubin G et al. The Aarhus statement: Improving design and reporting of studies on early cancer diagnosis. Br J Cancer 2012; 106: 1262-1267.
Conway D I, Purkayastha M, Chestnutt I G. The changing epidemiology of oral cancer: definitions, trends, and risk factors. Br Dent J 2018; 225: 867-873.
Healthcare Improvement Scotland. Referral Guidelines for Suspected Head and Neck Cancer. 2019. Available at http://www.cancerreferral.scot.nhs.uk/head-and-neck-cancers (accessed June 2020).
Cancer Research UK. Oral cancer recognition toolkit. 2015. Available online at https://www.doctors.net.uk/eClientopen/CRUK/oral_cancer_toolkit_2015_open/referral-decision-aid.html (accessed June 2020).
Acknowledgements
There is no funding to declare for this study. We would like to acknowledge and thank Mrs Sadie Khwaja, ENT specialist, Stepping Hill Hospital, Manchester, UK, for her help in preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors have no declarations of interest.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0.© The Author(s), under exclusive licence to British Dental Association 2020
About this article
Cite this article
Wilcock, J., Grafton-Clarke, C. Diagnostic intervals in oropharyngeal cancers from a primary care perspective: a ten-year case note review. Br Dent J (2021). https://doi.org/10.1038/s41415-021-2947-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41415-021-2947-6
- Springer Nature Limited