Abstract
Autologous(auto-) and allogeneic(allo-) hematopoietic stem cell transplantation (HSCT) are key treatments for relapsed/refractory diffuse large B-cell lymphoma (DLBCL), although their roles are challenged by CAR-T-cells and other immunotherapies. We examined the transplantation trends and outcomes for DLBCL patients undergoing auto-/allo-HSCT between 1990 and 2021 reported to EBMT. Over this period, 41,148 patients underwent auto-HSCT, peaking at 1911 cases in 2016, while allo-HSCT saw a maximum of 294 cases in 2018. The recent decline in transplants corresponds to increased CAR-T treatments (1117 cases in 2021). Median age for auto-HSCT rose from 42 (1990–1994) to 58 years (2015–2021), with peripheral blood becoming the primary stem cell source post-1994. Allo-HSCT median age increased from 36 (1990–1994) to 54 (2015–2021) years, with mobilized blood as the primary source post-1998 and reduced intensity conditioning post-2000. Unrelated and mismatched allo-HSCT accounted for 50% and 19% of allo-HSCT in 2015–2021. Three-year overall survival (OS) after auto-HSCT improved from 56% (1990–1994) to 70% (2015–2021), p < 0.001, with a decrease in relapse incidence (RI) from 49% to 38%, while non-relapse mortality (NRM) remained unchanged (4%). After allo-HSCT, 3-year-OS increased from 33% (1990–1999) to 46% (2015–2021) (p < 0.001); 3-year RI remained at 39% and 1-year-NRM decreased to 19% (p < 0.001). Our data reflect advancements over 32 years and >40,000 transplants, providing insights for evaluating emerging DLBCL therapies.
Similar content being viewed by others
Introduction
The first reports on autologous and allogeneic transplantation of hematopoietic stem cells performed in end-stage patients with relapsed or refractory aggressive lymphoma were published in the early 1990s [1,2,3,4,5,6]. The basic principles of transplantation first described in patients with leukemia were shown to also apply to patients with lymphoma: the superior anti-tumor effect of dose-escalated chemotherapy to prepare patients for transplantation, better outcomes of patients with chemo-sensitive as compared to chemo-refractory disease, and the favorable survival of patients transplanted in complete or partial remission as opposed to patients being refractory to salvage chemotherapy [7,8,9]. Results of allogeneic bone marrow transplantation suggested the existence of a graft-versus-lymphoma effect (GvL) comparable to the graft-versus-leukemia effect described earlier [10]. The albeit limited success of allo-HSCT in patients with completely chemorefractory disease and the ability of donor lymphocyte infusions (DLI) to induce further remissions in patients relapsing after allo-HSCT have repeatedly been taken as an evidence of GvL [11,12,13,14,15]. Further relying on the therapeutic potential of donor T-cells, conditioning shifted from myeloablative conditioning (MAC) to reduced-intensity conditioning (RIC) especially in patients with chemosensitive disease and low tumor burden [12, 13, 16,17,18,19]. RIC also resulted in expanded access to allo-HSCT for older and frail patients [12, 13, 17]. The switch from bone marrow to G-CSF-mobilized blood as the preferred stem cell source, first reported for auto-HSCT [20, 21] and later for allo-HSCT [22], also contributed to the steep increase of transplant numbers observed for all lymphomas in the new millennium [23]. Large donor registries and the adoption of haplo-identical transplantation helped finding a suitable donor for almost every patient within a short period of time. Unfortunately, despite all progress made in donor-recipient matching, conditioning, GvHD prophylaxis, and supportive care, allo-HSCT remains loaded with a relatively high treatment-related mortality (TRM), primarily due to graft-versus-host disease [13, 16].
In recent years, transplantation of B-cell lymphoma patients has come under scrutiny by the development of CD19-directed chimeric antigen receptor (CAR) T-cells which show promising efficacy without the high TRM typical for allo-HSCT [24,25,26,27].
Here, we describe the pivotal changes in transplant modalities and outcomes of auto- and allo-HSCT for diffuse large B-cell lymphoma (DLBCL) in a very large cohort of patients registered with the European Society for Blood and Marrow Transplantation (EBMT) over 32 years. These benchmarking data will help to assess new therapeutic strategies for patients with DLBCL.
Methods
Data collection
We conducted a retrospective analysis of patients registered with the EBMT. Details describing the data collection process, quality management, and data hosting have been published previously [28]. All participating institutions are required to obtain written informed consent from patients prior to registration with EBMT, following the current version of the Helsinki Declaration. Numbers of auto-HSCT, allo-HSCT, and CAR T-cell infusions performed in adult patients with DLBCL between 1990 and 2021 were collected from centers reporting to the EBMT. For CAR T-cells, numbers were available from 2016 to 2021. Adult patients ≥18 years diagnosed with DLBCL receiving auto-HSCT as a first transplant or allo-HSCT as first transplant or after previous auto-HSCT, grafted between 1990 and 2021 were identified in the EBMT registry. For this analysis, patients with DLBCL were considered, including germinal center B-cell type (GCB) DLBCL, activated B-cell type (ABC or non-GCB) DLBCL, DLBCL not otherwise specified (NOS), primary cutaneous DLBCL, EBV-positive DLBCL, DLBCL associated with chronic inflammation, intravascular large B-cell lymphoma, ALK-positive large B-cell lymphoma, Human Herpesvirus-8 (HHV8) positive DLBCL, NOS as well as additional DLBCL-related subtypes as shown in Fig. S1. Eligible patients were registered by 578 transplant centers. Over the 32-year period from 1990 to 2021, the process of data reporting to EBMT evolved in accordance with national and center-specific regulations. EBMT members are generally required to report all cases, but consistent data reporting is the responsibility of individual centers. Consequently, we are unable to report to which extent the number of cases reported to EBMT reflects the number of cases actually transplanted in the countries covered by our report and how the number of reported compared to actually transplanted cases may have changed over time. Centers contributing 200 or more patients are listed in Table S1.
Definitions
Diagnosis of DLBCL was based on local pathology review using criteria effective at the time of diagnosis. Disease stage was classified according to the Ann Arbor staging system. Refractory disease was defined as disease progressing during first-line (immuno-)chemotherapy or in patients with transient response [complete (CR) or partial response (PR) lasting ≤3 months] after induction treatment. Relapse was diagnosed in case of lymphoma recurrence occurring at least 3 months after end of therapy in patients having achieved CR. Disease status was assessed by individual investigators according to standard criteria at the time patients were referred for transplantation and classified as CR, PR, stable disease (SD) and progressive disease (PD). Regimens containing TBI > 6 Gy, total oral busulfan >8 mg/kg or a total of intravenous busulfan >6.4 mg/kg body weight were classified as myeloablative, all other regimens were classified as reduced intensity conditioning (RIC) [29]. The diagnosis and grading of acute GvHD (aGvHD) and chronic GvHD (cGvHD) were made by transplant centers according to established criteria [30, 31].
Statistical analysis
Endpoints analyzed were progression-free survival (PFS) defined as survival without lymphoma relapse or progression, overall survival (OS) defined as time from transplantation to death from any cause; non-relapse mortality (NRM) defined as death without previous lymphoma relapse and relapse incidence (RI) defined as disease recurrence after transplantation. In patients receiving allo-HSCT, the incidence and severity of aGvHD and cGvHD were analyzed. All outcomes were measured from the day of transplantation. Surviving patients were censored at the time of last contact. The probabilities of OS and PFS were calculated using the Kaplan–Meier method. We calculated cumulative incidences for RI and NRM using a competing risk model, where death was treated as a competing event for relapse. Death and relapse were considered as competing events for aGvHD and cGvHD. Demographics were compared between groups using the chi- squared test or Fisher’s exact test for categorical variables and the Mann–Whitney U test for continuous variables. Univariate analyses were performed using the log-rank test for PFS and OS, while Gray’s test was used for competing risk outcome data. Multivariate analyses were performed using the Cox proportional-hazards regression model. Results were reported as hazard ratios (HR) with a 95% confidence interval (95% CI). All statistical tests were two-sided with a type I error fixed at 0.05 for factors associated with time-to-event outcomes. All analyses were performed using R version 4.3.3 with the R packages survival version 3.5-8, cmprsk version 2.2-11 and Hmisc version 5.1-2. (R Core Team. R: a language for statistical computing. 2014. R Foundation for Statistical Computing, Vienna, Austria).
Results
Evolution of transplantation and CAR T-cell numbers over time
In total, 41,148 auto-HSCTs and 4562 allo-HSCTs for DLBCL meeting the inclusion criteria were registered with EBMT between 1990 and 2021. As shown in Fig. 1A, transplantation activities constantly increased over time. Annual numbers of auto-HSCT increased from 92 in 1990 to reach a maximum of 1911 in 2016; allo-HSCT increased from 5 in 1990 to 294 in 2018. After 2018, the numbers of auto-HSCT and allo-HSCT sharply declined with no more than 1503 and 192 HSCT reported for 2021, respectively. Conversely, the number of CAR T-cell infusions steeply increased since 2016 (3 CAR T-cell therapies reported to EBMT) to 1117 in 2021 (Fig. 1A).
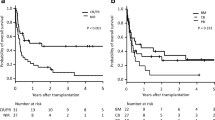
Numbers of auto- and allo-HSCT (A) by indicated periods between 1990 and 2021. The proportion (B) of patients undergoing auto-HSCT and allo-HSCT in CR/PR or SD/PD. Auto-/allo-HSCT autologous/allogeneic hematopoietic stem cell transplantation, CR complete remission, PR partial remission, SD stable disease, PD progressive disease.
Detailed clinical data were available for 43,260 DLBCL patients, of which 41,148 received an auto-HSCT and 4562 underwent allo-HSCT (2450 patients received allo-HSCT after auto-HSCT). The number of reporting centers as well as the number of patients per center increased over time. The number of patients receiving auto-HSCT in CR or PR increased from 681 (71%) between 1990-1994 to 11,287 patients in the most recent period accounting for 91% of all auto-HSCT (Fig. 1B). Similar trends were observed for patients undergoing allo-HSCT.
Auto-HSCT: patient characteristics and outcomes
The age and the proportion of patients with good performance status undergoing auto-HSCT significantly increased from 42 years (range: 18–67) to 58 years (range: 49–65) (p < 0.001) and from 90% to 94% (p < 0.001) for the time periods 1990–1994 and 2015–2021, respectively (Table 1). After 1994, peripheral blood (PB) emerged as the universally used stem cell source, accounting for more than 97% of all auto-HSCT between 1990 and 2021. TBI for conditioning has practically been abandoned after 2005 (Table 1). Preparation with BEAM or similar regimens remained most popular throughout all time periods (Table 1). Rituximab as part of conditioning was first reported after 2000 with 7.2% of all patients receiving Rituximab between 2015 and 2021 (Table 1). Significantly higher proportions of patients in CR or PR than in SD or PD were autografted over time (p < 0.001) (Fig. 1B, Table 1).
Major outcomes of patients receiving auto-HSCT are shown in Fig. 2 and Table 2. With a median follow-up of 4.7 years (95% CI: 4.7–4.8 years), 1-year, 5-year, and 10-year overall survival (OS) rates were 78.6% (95% CI: 78.2–79.1%), 61.1% (95% CI: 60.4–61.7%), and 52.6% (95% CI: 51.8–53.4%). We noted significant improvements in OS and PFS over time reaching 3-year OS and PFS rates of 69.5% (95% CI: 68.4–70.5%) and 55.9% (95% CI: 54.8–57.0%) for the 2015-2021 period (Table 2). Three-year relapse rates significantly decreased form 49.4% (95% CI: 46–52.7) for the 1990-1994 period to 38.0% (95% CI: 36.9–39.0%) in the 2015-2021 period. One-year NRM remained at 4–6% throughout the entire study period despite slight improvements over time (Fig. 2, Table 2).
Figure S2A illustrates outcomes of patients who underwent auto-HSCT for consolidation after achieving a CR following one treatment line compared to two and more treatment lines. Two-year OS and PFS of patients transplanted after one treatment line were significantly better than in patients undergoing auto-HSCT after two or more lines of therapy [3-year OS: 79.3% (95% CI: 77.8–80.8%) vs. 72.6.7% (95% CI: 71.1–74.1%) vs. 69.1% (95% CI: 66.2–71.1%); 3-year PFS: 68.3% (95% CI: 66.5–70.1%) vs. 58.6% (95% CI: 56.9–60.3%) vs. 52.0% (95% CI: 48.9–55.0%); p < 0.001 for both]. This difference was primarily due to a lower relapse incidence in patients who underwent consolidative auto-HSCT (p < 0.001). As shown in Fig. S2B, patients who underwent auto-HSCT not being in CR had significantly inferior OS and PFS compared to CR patients after auto-HSCT (both p < 0.001).
To further investigate the effect of remission status and conditioning before auto-HSCT, we used a multivariate model including the following variables with known prognostic relevance: year of HSCT, age at HSCT, gender, time interval from HSCT ≤ 12 months, disease status at HSCT, and type of conditioning regimen (Table S2). For Auto-HSCT patients, not being in CR was associated with lower OS and PFS rates, as well as being treated before 2000 compared to after 2000, similarly a longer interval from diagnosis to HSCT ( > 12 months) was associated with inferior OS and PFS compared to patients transplanted within 12 months from diagnosis (all p < 0.001) (Table S2). These differences in OS and PFS were primarily influenced by an increased relapse risk in patients transplanted not in CR (p < 0.001), patients who underwent auto-HSCT before 2000 [1995–1999 period: HR = 1.25 (95% CI: 1.14–1.37) compared to 2015–2021 period as reference, p < 0.001], as well as receiving conditioning therapy other than BEAM [HR = 1.11 (95% CI: 1.06–1.17), p < 0.001], or a longer treatment interval from diagnosis ( > 12 months) [HR = 1.20 (95% CI: 1.15–1.26), p < 0.001].
Allo-HSCT: patient characteristics and outcomes
Major clinical characteristics of allografted patients are shown in Table 3. Patient age significantly increased from 36 years (range: 19–50) in 1990–1994 to 54 years (range: 18–76) in 2015–2021 (p < 0.001). The median time from diagnosis to allo-HSCT increased from 15 months (range 3–74) for the 1990–1994 period to 19 months (range 1–354) (p < 0.001) over time with an increasing proportion of patients having failed a previous auto-HSCT (47% of all allo-HSCT for 2015–2021) (Table 3). Peripheral blood became the universal source of allogeneic stem cells after 1998. The proportion of patients undergoing allo-HSCT in CR/PR significantly increased from 69% in 1990–1994 to 80% in 2015–2021 (p < 0.001) (Table 3). RIC was introduced in the 1990s and preceded allo-HSCT in 54% of cases in the most recent time period. In the early days, 74% of patients received TBI as part of conditioning declining to 23% in the most recent period (Table 3). Transplantations from unrelated or haploidentical/mismatched related donors increased over time; 50% and 19% of HSCT were from such donors between 2015 and 2021. The frequencies for T-cell depletion and other GVHD prophylaxis are listed in Table 3.
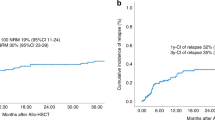
Figure 3 and Table 4 demonstrate the improvement of key outcome parameters. With a median follow-up of 5.2 years (95% CI: 5.0–5.5 years), 3-year OS- and PFS-rates of 46.1% (95% CI: 43.6–48.6%) and 38.5% (95% CI: 36.0–41.0%) were noted for the 2015–2021 period. For the entire study period, 1-year, 5-year, and 10-year OS rates were 54.5% (95% CI: 53.0–56.0), 37.8% (95% CI: 35.8–39.7%), 30.6% (95% CI: 28.1–33.1%); PFS was 45.4% (95% CI: 43.8–47.0%), 31.4% (95% CI: 29.5–33.4%), 24.7% (95% CI: 22.3–27.2%), respectively. The observed improvements in OS and PFS rates can partially be explained by a significant decrease of cumulative relapse incidences (Table 4, Fig. 3). Main causes of death after allo-HSCT were disease-related/relapse in 44.7% of cases followed by transplant--related causes, mostly infectious complications (35.8%), GvHD (12.9%), other/unknown causes (7.1%) and secondary malignancies (0.3%). We observed a significant decrease in NRM after 1999 (Table 4, Fig. 3). For acute and chronic GvHD incidences, a significant decrease over time was noted (Table 4, Fig. S3). No significant differences between matched-related (sibling and non-sibling) and unrelated donor transplants in terms of severe acute GvHD Grades III-IV at day 100 [8.2% (95% CI: 6.9–9.7%) vs. 8.5% (95% CI: 7.2–10.0%), p = 0.926] were observed. The incidences of chronic GvHD at 1 year were also comparable for patients with matched-related and unrelated donors with 24.1% (95% CI: 22.0–26.3%) and 23.2% (95% CI: 21.1–25.3%) (p = 0.833), respectively. Haploidentical/mismatched-related transplantations became more common since 2010 ( > 5% of allo-HSCT) with incidences of acute GvHD grades III–IV at day 100 being 12.6% (95% CI: 9.4–16.2%) and 16.7% (95% CI: 13.1–20.7%) for chronic GvHD at 1 year, comparing favorably to patients who received matched-related or unrelated donor transplants [acute GvHD grades III–IV at day 100: 8.4% (95% CI: 7.4–9.4%), p = 0.027; chronic GVHD at 1 year: 23.7% (95% CI: 22.2–35.2%), p = 0.002]. To evaluate the effect of disease status and conditioning intensity we used a multivariate model that incorporated year of HSCT, age at HSCT, gender, time interval from diagnosis to HSCT ≤ 12 months, disease status at HSCT, and conditioning intensity (Table S3). This model revealed that disease status at allo-HSCT, most notably CR, as well as a longer interval between diagnosis and HSCT ( > 12 months) were associated with superior OS and PFS rates. These observations reflect a higher risk of relapse for patients not in CR at allo-HSCT (p < 0.001) and a reduced risk of relapse in patients allografted >12 months from diagnosis [HR = 0.75 (95% CI: 0.65–0.85), p < 0.001]. Conditioning with MAC protocols was associated with inferior PFS compared to RIC conditioning [HR = 1.23 (95% CI: 1.12–1.34), p < 0.001] and OS [HR = 1.34 (95% CI: 1.22–1.47), p < 0.001] in the model. The risk of NRM significantly increased with age at allo-HSCT [HR = 1.12 (95% CI: 1.08–1.15), p < 0.001], and conditioning intensity [HR = 1.32 (95% CI: 1.14–1.53), p < 0.001] (Table S3).
Overall survival (A), progression-free survival (B), cumulative incidence of relapse (C) and non-relapse mortality (D) after allo-HSCT by indicated periods between 1990 and 2021 (outcomes for the 1990–1999 period are summarized due to low event numbers between 1990 and 1994). Allo-HSCT allogeneic hematopoietic stem cell transplantation.
Discussion
In the current study, we describe the evolution of transplantation activities and modalities, and provide outcome data for more than 40,000 transplants performed for DLBCL covering 32 years. We observed steadily increasing numbers of autologous and allogeneic HSCT with a growing proportion of transplants being performed in CR or PR. After 2018, a steep decline in transplantation activities was observed coinciding with the availability of CAR T-cells and other new agents entering the clinical arena. Although we cannot exclude that the SARS-CoV-2 pandemic temporarily disrupted transplant activities also for DLBCL, there is evidence based on the most recent numbers reported to EBMT that post-pandemic transplant numbers continue to decrease [32, 33]. The promising reports on CAR T-cell therapies including the recently published randomized studies comparing CAR T-cells to auto-HSCT for treatment of DLBCL patients failing first-line therapy will further boost CAR T-cell therapy [24, 26]. The approval of bispecific antibodies and antibody-drug-conjugates will accelerate this trend [34,35,36].
Clinical characteristics of transplant patients evolved from younger patients with advanced disease towards an older but medically fit population undergoing transplantation in CR or PR. Changes in transplant modalities like the use of mobilized blood as the source of hematopoietic stem cells for auto- and allo-HSCT and the adoption of RIC prior to allo-HSCT fostered easier access to transplantation also for older and frail patients. The switch from HLA-identical sibling donors to unrelated, and, more recently, haploidentical donors paralleled by changes in GvHD prophylaxis also contributed to the increase in allogeneic transplantation numbers.
Until today, there is no consensus on the optimal preparatory regimen for auto- or allo-HSCT [12, 13, 16, 17, 37,38,39]. BEAM remains the most commonly used conditioning regimen prior to auto-HSCT, while busulfan/fludarabine and TBI-based protocols were most frequently used to prepare for allo-HSCT. TBI-based protocols using doses >6 Gy clearly decreased from 75% in the 1990s to 9% in the most recent period, underscoring the transition to mainly chemotherapy-based RIC. The most popular MAC protocols combined busulfan, fludarabine and TBI, while the most popular RIC regimens were fludarabine/melphalan and busulfan/fludarabine at lower doses. Because patients prepared with MAC tend to present with higher tumor burden and more advanced disease, direct comparisons of outcomes after RIC and MAC remain problematic. The reported 3-year NRM rate of approximately 24% aligns with previous findings that myeloablative conditioning (MAC) regimens are associated with higher NRM [16, 40, 41]. Despite the increasing use of RIC over time, in this analysis, over 40% of allo-HSCTs performed since 2010 were preceded by MAC conditioning. This likely has contributed to the relatively high NRM rates observed [42, 43]. The increasing usage of RIC regimens over time was also paralleled by more patients undergoing allo-HSCT in CR or PR and reflects the investigators’ views that RIC is the preferred conditioning approach for patients with less rapidly growing tumors and lower tumor burden at transplantation. For older and frail patients, RIC frequently is the only option to prepare patients for allo-HSCT. OS and PFS as the most important outcome parameters significantly improved over time. This seems at least partly due to a decrease in relapse rates, which in turn mirrored the lower number of patients transplanted with active disease. The 3-year OS- and PFS rates of approximately 45% and 38% for the most recent period appear slightly better than those reported for the only randomized trial involving allo-HSCT for DLBCL (42% and 39%, respectively) [16] but are in line with other registry-based analyses, which consistently showed 3-year PFS rates of 30–40% [13, 41, 44].
NRM rates after allo-HSCT and auto-HSCT showed a trend to decline after 1999. Improvements in recipient/donor matching, conditioning, GvHD prophylaxis and supportive care obviously were able to compensate for the increasing patient age and broader donor selection. Patients who received an auto-HSCT for consolidation of a first CR or PR showed the highest survival rates approaching 80%. Despite such excellent outcomes, consolidative auto-HSCT cannot generally be recommended after several randomized studies failed to demonstrate an improvement of PFS or OS in young, high-risk patients with DLBCL when compared to conventional immunochemotherapy [45,46,47].
In the early days, a large proportion of allo-HSCT were performed in patients with active disease conditioned with myeloablative regimens [48, 49]. These transplants were characterized by high relapse and NRM rates resulting in poor survival. In recent years, allo-HSCT frequently was performed in responding patients after reduced-intensity conditioning (RIC) featuring lower NRM and RI resulting in better survival [12, 13]. A large proportion of patients underwent allo-HSCT after failing auto-HSCT with results comparable to those reported for patients without a previous autograft [13, 49].
The observed parallel increase in GvHD prophylaxis using post-transplantation cyclophosphamide (PTCy) and haploidentical donors after 2015 is likely attributable to encouraging results highlighting comparable survival rates for haploidentical donors as compared to matched sibling or unrelated donors [50,51,52]. Recently, a large phase 3 trial demonstrated superior outcomes with PTCy-based GvHD prophylaxis in patients, predominantly with leukemia/MDS ( > 85%), undergoing allo-HCT after reduced-intensity conditioning (RIC) with either an HLA-matched or 7/8-mismatch donor. Although these findings may apply also to DLBCL patients, no formal proof is available and studies addressing this point are unlikely to be conducted, given the recent decline of allo-HCT in patients with DLBCL [53].
The present study has limitations. Most importantly, we cannot know how many patients initially deemed transplant candidates could not make it to transplantation because of disease progression or toxicity of salvage therapies. Unknown confounders of outcomes after transplantation might have gone undetected, and we cannot directly compare outcomes after HSCT with outcomes of patients receiving alternative treatments. Additionally, due to changes in data reporting over time not all details of pre-transplant treatments may have been caught, particularly for patients transplanted in the early years of data collection. While we acknowledge these and other limitations of any retrospective analysis, we believe that the huge number of patients closely followed for up to 32 years provides a solid foundation for further discussion of evolving treatment strategies in DLBCL.
Our analysis reflects the changing role of transplantation in the treatment of DLBCL. Allo-HSCT, CAR-T cells, and bispecific antibodies represent different forms of immunotherapy. Therefore, we suggest that international databases like ours should be open to include all these modalities, hopefully also allowing for comparison of competing strategies. The large body of data presented here summarizes long-term outcomes after auto- and allo-HSCT, which may serve as real-world benchmarks when comparing transplantation to newer, more targeted therapies.
Data availability
The datasets presented in the study are included in the article/Supplementary information. Further inquiries can be directed to the corresponding author.
References
Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N. Engl J Med. 1995;333:1540–5.
Ratanatharathorn V, Uberti J, Karanes C, Abella E, Lum LG, Momin F, et al. Prospective comparative trial of autologous versus allogeneic bone marrow transplantation in patients with non-Hodgkin’s lymphoma. Blood. 1994;84:1050–5.
Bosly A, Coiffier B, Gisselbrecht C, Tilly H, Auzanneau G, Andrien F, et al. Bone marrow transplantation prolongs survival after relapse in aggressive-lymphoma patients treated with the LNH-84 regimen. J Clin Oncol. 1992;10:1615–23.
Vose JM, Anderson JR, Kessinger A, Bierman PJ, Coccia P, Reed EC, et al. High-dose chemotherapy and autologous hematopoietic stem-cell transplantation for aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 1993;11:1846–51.
Chopra R, Goldstone AH, Pearce R, Philip T, Petersen F, Appelbaum F, et al. Autologous versus allogeneic bone marrow transplantation for non-Hodgkin’s lymphoma: a case-controlled analysis of the European Bone Marrow Transplant Group Registry data. J Clin Oncol. 1992;10:1690–5.
Reece DE, Barnett MJ, Connors JM, Fairey RN, Fay JW, Greer JP, et al. Intensive chemotherapy with cyclophosphamide, carmustine, and etoposide followed by autologous bone marrow transplantation for relapsed Hodgkin’s disease. J Clin Oncol. 1991;9:1871–9.
Crump M, Kuruvilla J, Couban S, MacDonald DA, Kukreti V, Kouroukis CT, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol. 2014;32:3490–6.
Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–90.
Oliansky DM, Czuczman M, Fisher RI, Irwin FD, Lazarus HM, Omel J, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of diffuse large B cell lymphoma: update of the 2001 evidence-based review. Biol Blood Marrow Transpl. 2011;17:20–47.e30.
Weiden PL, Flournoy N, Thomas ED, Prentice R, Fefer A, Buckner CD, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N. Engl J Med. 1979;300:1068–73.
Bishop MR, Dean RM, Steinberg SM, Odom J, Pavletic SZ, Chow C, et al. Clinical evidence of a graft-versus-lymphoma effect against relapsed diffuse large B-cell lymphoma after allogeneic hematopoietic stem-cell transplantation. Ann Oncol. 2008;19:1935–40.
Thomson KJ, Morris EC, Bloor A, Cook G, Milligan D, Parker A, et al. Favorable long-term survival after reduced-intensity allogeneic transplantation for multiple-relapse aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27:426–32.
van Kampen RJ, Canals C, Schouten HC, Nagler A, Thomson KJ, Vernant JP, et al. Allogeneic stem-cell transplantation as salvage therapy for patients with diffuse large B-cell non-Hodgkin’s lymphoma relapsing after an autologous stem-cell transplantation: an analysis of the European Group for Blood and Marrow Transplantation Registry. J Clin Oncol. 2011;29:1342–8.
Urbano-Ispizua A, Pavletic SZ, Flowers ME, Klein JP, Zhang MJ, Carreras J, et al. The Impact of Graft-versus-Host Disease on the Relapse Rate in Patients with Lymphoma Depends on the Histological Subtype and the Intensity of the Conditioning Regimen. Biol Blood Marrow Transpl. 2015;21:1746–53.
Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–5.
Glass B, Hasenkamp J, Wulf G, Dreger P, Pfreundschuh M, Gramatzki M, et al. Rituximab after lymphoma-directed conditioning and allogeneic stem-cell transplantation for relapsed and refractory aggressive non-Hodgkin lymphoma (DSHNHL R3): an open-label, randomised, phase 2 trial. Lancet Oncol. 2014;15:757–66.
Rezvani AR, Norasetthada L, Gooley T, Sorror M, Bouvier ME, Sahebi F, et al. Non-myeloablative allogeneic haematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: a multicentre experience. Br J Haematol. 2008;143:395–403.
Khouri IF, Keating M, Korbling M, Przepiorka D, Anderlini P, O’Brien S, et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol. 1998;16:2817–24.
McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–400.
Schmitz N, Linch DC, Dreger P, Goldstone AH, Boogaerts MA, Ferrant A, et al. Randomised trial of filgrastim-mobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. Lancet. 1996;347:353–7.
Beyer J, Schwella N, Zingsem J, Strohscheer I, Schwaner I, Oettle H, et al. Hematopoietic rescue after high-dose chemotherapy using autologous peripheral-blood progenitor cells or bone marrow: a randomized comparison. J Clin Oncol. 1995;13:1328–35.
Schmitz N, Dreger P, Suttorp M, Rohwedder EB, Haferlach T, Loffler H, et al. Primary transplantation of allogeneic peripheral blood progenitor cells mobilized by filgrastim (granulocyte colony-stimulating factor). Blood. 1995;85:1666–72.
Stem Cell Trialists’ Collaborative G. Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005;23:5074–87.
Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N. Engl J Med. 2022;386:640–54.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl J Med. 2017;377:2531–44.
Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N. Engl J Med. 2022;386:629–39.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl J Med. 2019;380:45–56.
Cwynarski K, van Biezen A, de Wreede L, Stilgenbauer S, Bunjes D, Metzner B, et al. Autologous and allogeneic stem-cell transplantation for transformed chronic lymphocytic leukemia (Richter’s syndrome): A retrospective analysis from the chronic lymphocytic leukemia subcommittee of the chronic leukemia working party and lymphoma working party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2012;30:2211–17.
Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transpl. 2009;15:367–9.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transpl. 2015;21:389–401.e1.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transpl. 1995;15:825–8.
Passweg JR, Baldomero H, Chabannon C, Corbacioglu S, de la Camara R, Dolstra H, et al. Impact of the SARS-CoV-2 pandemic on hematopoietic cell transplantation and cellular therapies in Europe 2020: a report from the EBMT activity survey. Bone Marrow Transpl. 2022;57:742–52.
Passweg JR, Baldomero H, Ciceri F, Corbacioglu S, de la Camara R, Dolstra H, et al. Hematopoietic cell transplantation and cellular therapies in Europe 2021. The second year of the SARS-CoV-2 pandemic. A Report from the EBMT Activity Survey. Bone Marrow Transpl. 2023;58:647–58.
Caimi PF, Ai W, Alderuccio JP, Ardeshna KM, Hamadani M, Hess B, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22:790–800.
Dickinson MJ, Carlo-Stella C, Morschhauser F, Bachy E, Corradini P, Iacoboni G, et al. Glofitamab for Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl J Med. 2022;387:2220–31.
Thieblemont C, Phillips T, Ghesquieres H, Cheah CY, Clausen MR, Cunningham D, et al. Epcoritamab, a Novel, Subcutaneous CD3xCD20 Bispecific T-Cell-Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial. J Clin Oncol. 2023;41:2238–47.
Gonzalez-Barca E, Boumendil A, Blaise D, Trneny M, Masszi T, Finel H, et al. Outcome in patients with diffuse large B-cell lymphoma who relapse after autologous stem cell transplantation and receive active therapy. A retrospective analysis of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transpl. 2020;55:393–9.
Chen YB, Lane AA, Logan B, Zhu X, Akpek G, Aljurf M, et al. Impact of conditioning regimen on outcomes for patients with lymphoma undergoing high-dose therapy with autologous hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2015;21:1046–53.
Jagadeesh D, Majhail NS, He Y, Ahn KW, Litovich C, Ahmed S, et al. Outcomes of rituximab-BEAM versus BEAM conditioning regimen in patients with diffuse large B cell lymphoma undergoing autologous transplantation. Cancer. 2020;126:2279–87.
Bento L, Gutierrez A, Novelli S, Montoro J, Pinana JL, Lopez-Corral L, et al. Allogeneic stem cell transplantation as a curative option in relapse/refractory diffuse large B cell lymphoma: Spanish multicenter GETH/GELTAMO study. Bone Marrow Transpl. 2021;56:1919–28.
Fenske TS, Ahn KW, Graff TM, DiGilio A, Bashir Q, Kamble RT, et al. Allogeneic transplantation provides durable remission in a subset of DLBCL patients relapsing after autologous transplantation. Br J Haematol. 2016;174:235–48.
Epperla N, Ahn KW, Khanal M, Litovich C, Ahmed S, Ghosh N, et al. Impact of reduced-intensity conditioning regimens on outcomes in diffuse large B cell lymphoma undergoing allogeneic transplantation. Transpl Cell Ther. 2021;27:58–66.
Ghosh N, Ahmed S, Ahn KW, Khanal M, Litovich C, Aljurf M, et al. Association of reduced-intensity conditioning regimens with overall survival among patients with non-hodgkin lymphoma undergoing allogeneic transplant. JAMA Oncol. 2020;6:1011–8.
Robinson SP, Boumendil A, Finel H, Blaise D, Poire X, Nicolas-Virelizier E, et al. Autologous stem cell transplantation for relapsed/refractory diffuse large B-cell lymphoma: efficacy in the rituximab era and comparison to first allogeneic transplants. A report from the EBMT Lymphoma Working Party. Bone Marrow Transpl. 2016;51:365–71.
Schmitz N, Nickelsen M, Ziepert M, Haenel M, Borchmann P, Schmidt C, et al. Conventional chemotherapy (CHOEP-14) with rituximab or high-dose chemotherapy (MegaCHOEP) with rituximab for young, high-risk patients with aggressive B-cell lymphoma: an open-label, randomised, phase 3 trial (DSHNHL 2002-1). Lancet Oncol. 2012;13:1250–9.
Cortelazzo S, Tarella C, Gianni AM, Ladetto M, Barbui AM, Rossi A, et al. Randomized Trial Comparing R-CHOP Versus High-Dose Sequential Chemotherapy in High-Risk Patients With Diffuse Large B-Cell Lymphomas. J Clin Oncol. 2016;34:4015–22.
Chiappella A, Martelli M, Angelucci E, Brusamolino E, Evangelista A, Carella AM, et al. Rituximab-dose-dense chemotherapy with or without high-dose chemotherapy plus autologous stem-cell transplantation in high-risk diffuse large B-cell lymphoma (DLCL04): final results of a multicentre, open-label, randomised, controlled, phase 3 study. Lancet Oncol. 2017;18:1076–88.
Peniket AJ, Ruiz de Elvira MC, Taghipour G, Cordonnier C, Gluckman E, de Witte T, et al. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transpl. 2003;31:667–78.
Doocey RT, Toze CL, Connors JM, Nevill TJ, Gascoyne RD, Barnett MJ, et al. Allogeneic haematopoietic stem-cell transplantation for relapsed and refractory aggressive histology non-Hodgkin lymphoma. Br J Haematol. 2005;131:223–30.
Ghosh N, Karmali R, Rocha V, Ahn KW, DiGilio A, Hari PN, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors versus HLA-matched sibling donors: a center for international blood and marrow transplant research analysis. J Clin Oncol. 2016;34:3141–9.
Martinez C, Gayoso J, Canals C, Finel H, Peggs K, Dominietto A, et al. Post-transplantation cyclophosphamide-based haploidentical transplantation as alternative to matched sibling or unrelated donor transplantation for hodgkin lymphoma: a registry study of the lymphoma working party of the european society for blood and marrow transplantation. J Clin Oncol. 2017;35:3425–32.
Dreger P, Sureda A, Ahn KW, Eapen M, Litovich C, Finel H, et al. PTCy-based haploidentical vs matched related or unrelated donor reduced-intensity conditioning transplant for DLBCL. Blood Adv. 2019;3:360–9.
Bolanos-Meade J, Hamadani M, Wu J, Al Malki MM, Martens MJ, Runaas L, et al. Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N. Engl J Med. 2023;388:2338–48.
Acknowledgements
We sincerely thank the cooperating centers reporting to the European Society for Blood and Marrow Transplantation (EBMT), as well as the local data managers and caregivers. For a detailed listing of centers that have made significant contributions to this study, please refer to Table S1. Most importantly, we owe our sincere gratitude to the patients who contributed their data.
Funding
The authors have reported no funding for the present study. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
P.B., B.G., A.S. and N.S. designed the study, interpreted data. P.B., A.S. and N.S. wrote the manuscript. P.B. M.F., M.N., H.F. J-E.G. collected data, performed statistical analyses, and interpreted the data. B.G. reviewed and edited the paper. All authors provided clinical data and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All participating institutions are required to obtain written informed consent from patients prior to registration with EBMT, following the current version of the Helsinki Declaration. The study was approved by the general assembly and review board of the LWP and complied with country-specific regulatory requirements.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Berning, P., Fekom, M., Ngoya, M. et al. Hematopoietic stem cell transplantation for DLBCL: a report from the European Society for Blood and Marrow Transplantation on more than 40,000 patients over 32 years. Blood Cancer J. 14, 106 (2024). https://doi.org/10.1038/s41408-024-01085-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-024-01085-9
- Springer Nature Limited