Abstract
People living with HIV and those diagnosed with alcohol use disorders (AUD) relative to healthy individuals commonly have low levels of serum albumin, substantiated as an independent predictor of cardiovascular events. White matter hyperintensities (WMH)—a neuroimaging feature of cerebral small vessel disease—are also related to cardiovascular disease. Despite consensus regarding associations between high levels of urine albumin and WMH prevalence, and low serum albumin levels and impaired cognitive functioning, relations between serum albumin and WMH burdens have rarely been evaluated. Here, a sample including 160 individuals with AUD, 142 living with HIV, and 102 healthy controls was used to test the hypothesis that serum albumin would be inversely related to WMH volumes and directly related to cognitive performance in the two diagnostic groups. Although serum albumin and periventricular WMH volumes showed an inverse relationship in both AUD and HIV groups, this relationship persisted only in the HIV group after consideration of traditional cardiovascular (i.e., age, sex, body mass index (BMI), nicotine use, hypertension, diabetes), study-relevant (i.e., race, socioeconomic status, hepatitis C virus status), and disease-specific (i.e., CD4 nadir, HIV viral load, HIV duration) factors. Further, serum albumin contributed more significantly than periventricular WMH volume to variance in performance on a verbal learning and memory composite score in the HIV group only. Relations in both HIV and AUD groups between albumin and hematological red blood cell markers (e.g., hemoglobin, hematocrit) suggest that in this sample, serum albumin reflects hematological abnormalities. Albumin, a simple serum biomarker available in most clinical settings, may therefore help identify periventricular WMH burden and performance levels in specific cognitive domains in people living with HIV. Whether serum albumin contributes mechanistically to periventricular WMH in HIV will require additional investigation.
Similar content being viewed by others
Introduction
People living with HIV relative to healthy control individuals commonly have low serum albumin levels that are prognostic for disease severity and rate of progression [1,2,3]. In individuals diagnosed with alcohol use disorders (AUD), low serum albumin levels are observed [4, 5] generally in the context of liver disease [6,7,8]. Additionally, individuals with HIV and AUD comorbidity have high rates of hepatitis C virus (HCV) infection [9,10,11], which can independently lower serum albumin [12,13,14,15,16,17].
Hypoalbuminemia (i.e., low serum albumin) occurs in liver cirrhosis, kidney disease, and malnutrition among other diagnoses due to mechanisms such as decreased liver synthesis, increased urinary excretion, or increased catabolism [18,19,20,21]. Low serum albumin levels can predict cardiovascular incidents (e.g., coronary artery disease, myocardial infarctions, heart failure, arrhythmias) and stroke after consideration of traditional risk factors such as body mass index (BMI), nicotine use, diabetes, and hypertension [22,23,24,25,26,27,28,29,30,31,32,33,34,35]. Low albumin may promote cardiovascular disease via decreased intravascular oncotic pressure, or mechanisms involving increased oxidative stress (i.e., decreased scavenging) or inflammation [36,37,38,39,40].
Cardiovascular disease plays a key role in the etiology of brain white matter hyperintensities (WMH) [41, 42]. Individuals infected with HIV [43,44,45] or diagnosed with AUD [46, 47] carry greater liability for cerebrovascular events, including WMH prevalence, than the general population [43, 48,49,50]. Indeed, WMH volumes enlarge at an accelerated rate in HIV relative to healthy controls [49, 51].
Since 2007, several studies have demonstrated relations between high urine albumin (i.e., albuminuria) and greater WMH prevalence independent of established risk factors (i.e., age, sex, hypertension, diabetes, nicotine use) [52] in both healthy and clinical populations [e.g., 1253 hypertensive participants, Mayo Clinic, MN, US [53]; 285 hypertensive participants, Nagoya, Japan [54]; 975 hypertensive participants, Barcelona, Spain [55]; 1215 healthy older (>60 years) adults, Seoul, Korea [56]; 2,671 healthy older (~75 years) adults, Reykjavik, Iceland [57]; 1,214 community dwelling adults, Fukuoka, Japan [58]; 112 at risk elderly (~68 years), Exeter, UK [59]; 5,324 patients taking oral antithrombotic agents across multiple sites in Japan [60]]. Surprisingly, despite this reproducible relationship between albuminuria and WMH prevalence and accruing evidence for associations between low serum albumin levels and cognitive impairment in a variety of clinical cohorts [e.g., 1,511 patients with heart failure, albumin<3.5 g/dL, multiple sites, Italy [61]; 1,284 adults ≥55 years, albumin=4.4±0.3 g/dL, Amsterdam, Netherlands [62]; 2,550 Chinese adults ≥55 years, albumin<4.0 g/dL, Singapore [63]; 433 hip fracture patients ≥65 years, albumin=3.51±0.47 g/dL, Israel [64]; 1,752 adults ≥65 years, albumin=3.9±0.4 g/dL, multiple sites, England [65]; 191 patients with Parkinson’s Disease, albumin levels unspecified, Pennsylvania, U.S. [66]; 274 patients with acute heart failure, albumin=3.4±0.4 g/dL, Osaka, Japan [67]], the correlation between serum albumin and WHM occurrence has rarely been explored. In 46 patients with systemic lupus erythematosus, low serum albumin (i.e., albumin=3.8±1.3 g/dL) was associated with larger WMH lesion volume [68]. By contrast, a study of 396 older (>55 years) healthy adults in Korea showed that low serum albumin was associated with high cerebral β amyloid reactivity but not with WMH volume [69].
Here, cross-sectional data comprising WMH volumes matched to clinical laboratory measures from 160 individuals with AUD, 142 infected with HIV, and 102 healthy controls were evaluated to test the hypothesis that low serum albumin levels would be associated with larger WMH volumes and worse cognitive performance in both diagnostic groups.
Methods
Participants
Cross-sectional neuroimaging and clinical laboratory data from 3 study groups (102 control, 160 AUD, 142 HIV) were extracted from a longitudinal dataset [51] drawn from published studies [51, 70, 71]. All but 18 of the 404 cases were from the first visit in longitudinal study; for these 18 cases, matching time points for blood and imaging markers were only available for the 2nd visit. Participants were recruited from local alcohol and drug recovery centers, HIV clinics, postcard mailings, recruitment flyers, and word of mouth. After obtaining written informed consent for study participation, approved by the SRI International and Stanford University School of Medicine Institutional Review Boards, volunteers underwent a Structured Clinical Interview for Diagnostic and Statistical Manual (DSM)-IV and DSM-5 Disorders (SCID) [72], structured health questionnaires, and a semi-structured timeline follow-back interview to quantify lifetime alcohol consumption [73].
Upon initial assessment, volunteers were excluded if they had a significant history of medical (e.g., liver cirrhosis, epilepsy, stroke, multiple sclerosis, uncontrolled diabetes, or loss of consciousness > 30 min), neurological (e.g., Parkinson’s disease), or psychiatric (e.g., schizophrenia, bipolar disorder) disorders other than an AUD (DSM-5). Other exclusionary criteria were substance dependence (other than alcohol for the AUD group) within the past 3 months or any other DSM disorder (for all groups). All participants also completed screening to ensure MRI safety and a breathalyzer test for recent alcohol consumption. Socioeconomic status (SES) was derived from the Four-Factor Index of Social Status, which considers education and occupation level and wherein a lower score reflects higher status [74]. Scores on the Alcohol Use Disorders Identification Test (AUDIT), developed by the World Health Organization as a self-report screening test to identify severity of AUD and provide an overall measure of hazardous drinking, were totaled (higher scores represent more intense drinking) [75,76,77]. Sitting blood pressure was collected twice. The mean of each of the two diastolic and systolic measures was used. As in other studies, the diagnostic groups relative to the healthy control group were less well-educated, had worse SES, and were more likely to include men, Black individuals, nicotine use, and HCV infection (Table 1) [70, 71, 78, 79].
Neuroimaging acquisition and analysis
Protocols and parameters
Scanning was conducted at SRI International on a GE Discovery MR750 system (Waukesha, WI, U.S.A.) with ASSET for parallel and accelerated imaging on an 8-channel head coil. Detection and localization of WMH used three magnetic resonance imaging (MRI) acquisition protocols: T1-weighted (T1-w) MRI for anatomical localization: 3D axial IR-Prep (inversion prepared) SPGR (SPoiled Gradient Recalled); repetition time (TR) = 6.5 ms, echo time (TE) = 1.54 ms, thickness (thick)=1.25 mm, locations (loc) =124, skip=0); T2-weighted (T2-w) MRI merged with T1-w data for skull stripping: 3D isotropic FSE (Fast Spin Echo; GE Name=CUBE), TR = 2500 ms, effective TE = 99 ms, echo train length (ETL) = 100 ms, thick=1 mm, loc=150, FOV = 256 mm, xy_matrix=256×256, Resolution=1 × 1 × 1 mm; and FLAIR (FLuid-Attenuated Inversion Recovery) imaging for estimates of WMH volumes: 2D axial, TR = 9000 ms, TE = 82.5 ms, inversion time (TI) = 2200 ms, thick=2.5 mm, loc=65.
MRI structural analysis
Preprocessing of T1-weighted SPGR data involved noise removal [80] and brain mask segmentation using FSL BET [81], AFNI 3dSkullStrip [82], and Robust Brain Extraction (ROBEX) [83] generating 3 brain masks. In parallel, noise-corrected, T1-weighted images were corrected for field inhomogeneity via N4ITK [84], brain masks were segmented [85], and the resulting segmented brain masks were reduced to one using majority voting [86]. Brain tissue segmentation (gray matter, white matter, and cerebrospinal fluid) of the skull-stripped T1-weighted images was generated via Atropos [84]. Parcellated maps of tissue used the parc116 atlas to define cortical (gray matter) and subcortical (gray and white matter) volumes summed for bilateral hemispheres.
WMH quantification
WMH analysis was accomplished with the “UBO Detector,” acluster-based, fully automated pipeline for extracting and calculating WMHs on a voxel basis [87]. This procedure yielded voxel maps for 2 WMH volumes: periventricular and deep. The pipeline required that FLAIR and T1-w data be warped into MNI space prior to non-rigid transformation into standard SRI atlas space. This was necessary for accurate placement of anatomical locations to enable comparisons across individuals and across imaging modalities on a voxel-wise basis without the need for further correction for differences in intracranial volume.
Blood sample collection
Blood samples were collected for analysis by Quest Diagnostics for complete blood count (CBC) (test code: 6399, CPT: 85025), comprehensive metabolic panel (test code: 10231, CPT code: 80053), and HIV and HCV screening with RNA quantification for seropositive individuals. CBC required whole blood collected in EDTA tubes; remaining tests used serum separator tubes (SST) tubes. The Quest Diagnostics reference range for serum albumin is 3.6–5.1 g/dL; levels ≤3.5 g/dL were considered below range [88].
To evaluate the significance of serum albumin, its relations with other blood biomarkers were considered. Relations between serum albumin and levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase (GGT), and alkaline phosphatase were used to evaluate liver disease; with eGFR and creatinine levels for kidney disease; with levels of vitamins B9 (folate) and B12 (cobalamin) for malnutrition; and with TNFα and IP10 levels for inflammation [for details on methods for cytokine measures see: [78, 89].
Cognitive composite scores
Cognitive composite scores matched to date of blood draw for each participant were extracted from an in-house laboratory release as described [51, 90]. Briefly, composites cognitive scores were created by averaging age-, education-, and sex-corrected Z-scores on performance on neuropsychological tests. Composites scores comprised tests of executive functioning, attention and working memory, visual and verbal learning, and visual and verbal memory as listed.
executive functioning
Trails B time or Color-trails time 2 +
Digit symbol raw score at 90 s or Symbol digit raw score at 90 s+
Phonological fluency (sum of unique “F” + “A” + “S” words)
attention and working memory
Trails A time or Color-trails time1 +
Wechsler Memory Scale-Revised (WMS-R) digits forward raw score total +
WMS-R digits backwards raw score total +
WMS-R block tapping forward total +
WMS-R block tapping backward total
visual and verbal learning
Rey-Osterrieth complex figure immediate raw score +
WMS-R logical memory immediate total
visual and verbal memory
Rey-Osterrieth complex figure delay raw score +
WMS-R logical memory delay total
Statistics
Statistics were performed using JMP® Pro 16.0.0 (SAS Institute Inc., Cary, NC, 1989-2021). For demographics, χ2 was used on categorical variables, Welch’s test for unequal variances was used on continuous variables. Significance required Bonferroni-corrected p-values as indicated in text or tables. Initial stepwise regression models for periventricular or deep WMH volumes selected from among 9 traditional cardiovascular risk variables [age, sex (male/female), race (black/white/other), BMI, SES, HCV status (positive/negative), nicotine use (never/past or current, self-report), hypertension (yes/no, yes = systolic ≥ 140 or diastolic ≥ 90), diabetes (yes/no, self-report)], serum albumin levels, and 3 disease specific markers per diagnoses (for AUD: total AUDIT score, lifetime alcohol consumed, AUD duration; for HIV: CD4 nadir, HIV viral load, HIV duration). These were followed by multiple regression analyses including only the variables selected by stepwise regressions. Performance in 4 cognitive domains also used multiple regressions. Factors were identified as significant contributors to variance in multiple regression models if they passed FDR (false discovery rate)-corrected logworth contribution to total variance. JMP uses Benjamini-Hochberg FDR p-value adjustment (defined as -log10[p-value]) to standardize p-values to permit comparison across multiple tests [91]. To confirm a priori selected variables were related, principal component analysis (PCA) on correlations were used to cluster variables. JMP iteratively splits clusters of variables and reassigns variables to clusters until no more splits are possible based on an algorithm developed by SAS [92].
Results
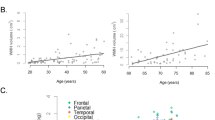
Serum albumin levels were low in the HIV relative to the AUD and healthy control groups (Fig. 1a). Collapsed across diagnosis groups, infection with HCV was also associated with low serum albumin levels (F = 26.0, p < 0.0001). The AUD (r = -0.25, p = 0.0016) and HIV (r = -0.23, p = 0.0071) groups showed an inverse relationship between serum albumin and periventricular WMH volume (Fig. 1b). Only the AUD group showed an inverse relationship between serum albumin and deep WMH volume (r = -0.20, p = 0.0159; Bonferroni-correction for 3 group comparisons, p-value = 0.05/3 = 0.0167; Fig. 1c). The HCV group (n = 88) did not show significant relations between serum albumin and periventricular (r = -0.12, p = 0.2728) or deep (r = -0.18, p = 0.0927) WMH volumes.
For periventricular WMH volume in the HIV group, the stepwise regression selected 4 (age, sex, BMI, and serum albumin, Fig. 2a) from among 13 (age, sex, race, SES, BMI, HCV status, nicotine use, presence of hypertension or diabetes, serum albumin levels, CD4 nadir, HIV viral load, years with HIV) variables. The follow-up multiple regression model in the HIV group including these 4 factors was significant (F4,141 = 11.0, p < 0.0001), explained 24.3% of the variance in periventricular WMH volumes, and was driven by age (p = 0.00004) with similar FDR significant contributions from sex (p = 0.005), serum albumin (p = 0.009), and BMI (p = 0.009, Fig. 2b).
In the AUD group, the stepwise regression identified 6 (i.e., age, sex, BMI, nicotine use, hypertension, and serum albumin) of 13 (age, sex, race, SES, BMI, HCV status, nicotine use, presence of hypertension or diabetes, serum albumin levels, total AUDIT score, lifetime alcohol consumed, years with AUD) variables as relevant to periventricular WMH volumes. The follow-up multiple regression in the AUD group including these 6 variables was significant (F6,159 = 9.1, p < 0.0001) and explained 26.4% of the variance in periventricular WMH volume; the only variable that contributed with FDR significance to the model, however, was age (p < 0.00001, Fig. 3a). For deep WMH volumes in the AUD group, the stepwise regression selected 3 (i.e., age, BMI, AUD duration) of the 13 variables The follow-up multiple regression in the AUD group was significant (F3,157 = 16.9, p < 0.0001) and explained 24.7% of the variance in deep WMH volumes; again, the only variable that contributed with FDR significance to the model was age (p < 0.00001, Fig. 3b).
Next, the contributions of serum albumin levels and periventricular WMH volumes to cognitive performance in 4 domains in the HIV group were evaluated. Serum albumin contributed with more FDR-weighted significance than WMH volume to performance in the visual and verbal memory domain (Table 2, Fig. 4). Similar analyses in the AUD group revealed comparable patterns not reaching significance (Table S1).
To confirm the salience of the identified relationships, a PCA was conducted in the combined HIV and AUD groups including the 9 variables that correlated with periventricular WM volumes (i.e., Fig. 2, 3: age, sex, albumin, BMI, nicotine use, hypertension, AUD duration; and visual and verbal memory performance). The PCA grouped the 9 variables into 4 clusters, the 2nd of which included serum albumin, periventricular WMH volumes, and the visual and verbal memory Z-score (Fig. 5).
To help interpret the biological significance of low serum albumin levels, correlates with other blood markers were evaluated separately in the HIV and AUD groups. Blood marker differences as a function of HCV were also evaluated as a positive control for liver disease. Whereas correlates of serum albumin (i.e., hemoglobin, hematocrit, mean corpuscular hemoglobin concentration, and prealbumin) overlapped in the HIV and AUD groups, blood marker correlates of HCV infection were different and included higher levels of aspartate aminotransferase (AST) and γ-glutamyl transferase (GGT) (Table 3). A PCA including albumin and the 14 blood markers associated with albumin or HCV demonstrated that whereas AST, GGT, IP10, TNF, and prealbumin clustered together, serum albumin clustered with red cell distribution width and mean corpuscular hemoglobin concentration (Fig. 6).
Discussion
The current results demonstrate that low serum albumin levels contribute significantly to explaining variance in periventricular WMH volumes in HIV after consideration of other relevant factors. Further, low serum albumin levels contributed more than periventricular WMH volumes to worse performance on tests of visual and verbal memory in HIV. Finally, relations between serum albumin and blood markers suggest that in this sample, low serum albumin levels reflect hematological abnormalities. Both albuminuria and hypoalbuminemia predict cardiovascular event prevalence independent of traditional risk factors, and albuminuria has consistently been shown to correlate with WMH volumes [52,53,54,55,56,57,58,59,60]. The current report, however, is among the first to evaluate the serum marker for its contribution to WMH volume. Only 2 relevant previous reports were identified: assessment in those with systemic lupus erythematosus [68] determined an inverse relationship between serum albumin and WMH volume; in healthy older adults, however, this correlation did not emerge [69] (also see, [93]). Our results are consistent with these prior observations as the correlation was salient in the diagnostic groups but not in the control cohort.
Initial analyses herein confirmed statistically significant, inverse relationships between lower serum albumin levels and greater periventricular WMH volumes in both HIV and AUD groups (i.e., Fig. 1b). In multivariate analyses considering traditional cardiovascular-, study-, and disease- related variables, however, this relation persisted only in the HIV group (i.e., Figs. 2 and 3). The lack of an enduring relationship between serum albumin and periventricular WMH volumes after consideration of covariates in the AUD group may be because they had neither liver disease or low serum albumin levels. This finding comports with the literature as the current sample was screened for known liver disease, and extant data indicates that serum albumin levels are low only in AUD complicated by liver disease [6,7,8].
It remains unclear why periventricular and deep WMH volumes have different biological correlates, but the current study is not the first to reveal differential relations. Indeed, several studies have reported unique correlates of periventricular versus deep WMH volumes [94,95,96]. For example, in a sample of 155 dementia-free older adults, hypertension was associated with periventricular WMH, whereas diabetes correlated with deep WMH volumes [97]. That hypertension [94, 97] and a decline in total cerebral blood flow [98] can be related to larger periventricular but not deep WMH volumes invites the speculation that serum albumin may mediate larger periventricular WMH volume by effects on blood pressure or perfusion.
Several studies in both healthy and clinical populations have reported associations between cognitive functioning and serum albumin levels [61, 63,64,65,66,67, 99,100,101,102,103,104,105,106]; some specifically noting relations between lower serum albumin and deteriorated memory performance [107]. Indeed, HIV mono-infected [108] and HIV + HCV co-infected [109] individuals with cognitive impairment, including disturbed visual memory in HIV + HCV-coinfection [110], have low serum albumin. The current report, however, may be the first to establish a relationship between lower serum albumin and worse cognitive functioning in an HIV mono-infected cohort.
Differences in blood marker correlates observed between HCV and serum albumin levels in AUD and HIV suggest that albumin functions more than just an indicator of liver status. That is, whereas both HCV and serum albumin correlated with serum markers of liver function (i.e., prealbumin), only serum albumin additionally correlated with hematological markers (i.e., lower hemoglobin, lower hematocrit). These relations between serum albumin and altered hemodynamic profiles have previously been reported [111] and may reflect cardiovascular dysfunction [112,113,114,115,116,117].
A limitation of the current study was access to serum but not also urine albumin levels. Had urine albumin been available, the relationship between these two measures observed in other populations [21, 118,119,120,121,122] could have been evaluated; and more might have been inferred about the biological role albumin plays in contributing to greater periventricular WMH volumes.
In conclusion, the current study contributes to a nascent literature demonstrating relations between serum albumin and WMH burden in clinical populations including those with HIV. Determination of whether low serum albumin contributes mechanistically via effects on vasculature, blood pressure, or perfusion [123,124,125] to periventricular WMH volumes will require additional investigation.
Data availability
Data described in the manuscript, code book, and analytic code will be made publicly and freely available without restriction at https://data.mendeley.com/.
References
Mehta SH, Astemborski J, Sterling TR, Thomas DL, Vlahov D. Serum albumin as a prognostic indicator for hiv disease progression. AIDS Res Hum Retroviruses. 2006;22:14–21. https://doi.org/10.1089/aid.2006.22.14.
Ronit A, Sharma S, Baker JV, Mngqibisa R, Delory T, Caldeira L, et al. Serum albumin as a prognostic marker for serious non-aids endpoints in the strategic timing of antiretroviral treatment (start) study. J Infect Dis. 2018;217:405–12. https://doi.org/10.1093/infdis/jix350.
Shah S, Smith CJ, Lampe F, Youle M, Johnson MA, Phillips AN, et al. Haemoglobin and albumin as markers of hiv disease progression in the highly active antiretroviral therapy era: Relationships with gender. HIV Med. 2007;8:38–45. https://doi.org/10.1111/j.1468-1293.2007.00434.x.
Hollstedt C, Dahlgren L. Peripheral markers in the female “hidden alcoholic”. Acta Psychiatr Scandinavica. 1987;75:591–6. https://doi.org/10.1111/j.1600-0447.1987.tb02840.x.
González-Reimers E, García-Valdecasas-Campelo E, Santolaria-Fernández F, de la Vega-Prieto MJ, Ros-Vilamajó R, Martínez-Riera A, et al. Pro-inflammatory cytokines in stable chronic alcoholics: Relationship with fat and lean mass. Food Chem Toxicol. 2007;45:904–9. https://doi.org/10.1016/j.fct.2006.11.012.
Torruellas C, French SW, Medici V. Diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20:11684–99. https://doi.org/10.3748/wjg.v20.i33.11684.
Marasini B, Agostoni A, Stabilini R, Dioguardi N. Serum proteins of hepatic and extrahepatic origin in alcoholic cirrhosis. Clin Chim Acta. 1972;40:501–2. https://doi.org/10.1016/0009-8981(72)90365-8.
Yamashiki N, Kawabata K, Murata M, Ikeda S, Fujimaki T, Suwa K, et al. Narrowing the patient-physician gap based on self-reporting and monthly hepatologist feedback for patients with alcohol-related liver disease: Interventional pilot study using a journaling smartphone app. JMIR Form Res. 2023;7:e44762 https://doi.org/10.2196/44762.
Fuster D, Sanvisens A, Bolao F, Rivas I, Tor J, Muga R. Alcohol use disorder and its impact on chronic hepatitis c virus and human immunodeficiency virus infections. World J Hepatol. 2016;8:1295–308. https://doi.org/10.4254/wjh.v8.i31.1295.
Lieber CS. Alcohol and hepatitis c. Alcohol Res Health. 2001;25:245–54.
Kim AY, Onofrey S, Church DR. An epidemiologic update on hepatitis c infection in persons living with or at risk of hiv infection. J Infect Dis. 2013;207:S1–6. https://doi.org/10.1093/infdis/jis927.
Chitturi S, George J. Predictors of liver-related complications in patients with chronic hepatitis c. Ann Med. 2000;32:588–91. https://doi.org/10.3109/07853890009002028.
Tillmann HL, Manns MP, Rudolph KL. Merging models of hepatitis c virus pathogenesis. Semin liver Dis. 2005;25:84–92. https://doi.org/10.1055/s-2005-864784.
Toshikuni N, Izumi A, Nishino K, Inada N, Sakanoue R, Yamato R, et al. Comparison of outcomes between patients with alcoholic cirrhosis and those with hepatitis c virus-related cirrhosis. J Gastroenterol Hepatol. 2009;24:1276–83. https://doi.org/10.1111/j.1440-1746.2009.05851.x.
Schley G, Köberle C, Manuilova E, Rutz S, Forster C, Weyand M, et al. Comparison of plasma and urine biomarker performance in acute kidney injury. PLOS ONE. 2015;10:e0145042 https://doi.org/10.1371/journal.pone.0145042.
Colombo M, McGurnaghan SJ, Blackbourn LAK, Dalton RN, Dunger D, Bell S, et al. Comparison of serum and urinary biomarker panels with albumin/creatinine ratio in the prediction of renal function decline in type 1 diabetes. Diabetologia. 2020;63:788–98. https://doi.org/10.1007/s00125-019-05081-8.
Krolewski AS. Progressive renal decline: The new paradigm of diabetic nephropathy in type 1 diabetes. Diab care. 2015;38:954–62. https://doi.org/10.2337/dc15-0184.
Fleck A, Hawker F, Wallace P, Raines G, Trotter J, Ledingham IM, et al. Increased vascular permeability: A major cause of hypoalbuminaemia in disease and injury. Lancet. 1985;325:781–4.
Barchel D, Almoznino-Sarafian D, Shteinshnaider M, Tzur I, Cohen N, Gorelik O. Clinical characteristics and prognostic significance of serum albumin changes in an internal medicine ward. Eur J Intern Med. 2013;24:772–8. https://doi.org/10.1016/j.ejim.2013.08.004.
Gatta A, Verardo A, Bolognesi M. Hypoalbuminemia. Intern Emerg Med. 2012;7:S193–9. https://doi.org/10.1007/s11739-012-0802-0.
Gounden V, Vashisht R, Jialal I. Hypoalbuminemia. Treasure Island (FL): StatPearls Publishing; 2023.
Phillips A, Shaper AG, Whincup P. Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet. 1989;334:1434–6.
Gillum RF, Ingram DD, Makuc DM. Relation between serum albumin concentration and stroke incidence and death: The nhanes i epidemiologic follow-up study. Am J Epidemiol. 1994;140:876–88. https://doi.org/10.1093/oxfordjournals.aje.a117176.
Goldwasser P, Feldman J. Association of serum albumin and mortality risk. J Clin Epidemiol. 1997;50:693–703. https://doi.org/10.1016/s0895-4356(97)00015-2.
Enia G, Mallamaci F, Benedetto FA, Panuccio V, Parlongo S, Cutrupi S, et al. Long‐term capd patients are volume expanded and display more severe left ventricular hypertrophy than haemodialysis patients. Nephrol Dialysis Transplant. 2001;16:1459–64. https://doi.org/10.1093/ndt/16.7.1459.
Feldman JG, Gange SJ, Bacchetti P, Cohen M, Young M, Squires KE, et al. Serum albumin is a powerful predictor of survival among hiv-1-infected women. J acquired immune Defic syndromes. 2003;33:66–73. https://doi.org/10.1097/00126334-200305010-00010.
Høstmark AT, Tomten SE. Serum albumin and self-reported prevalence of stroke: A population-based, cross-sectional study. Eur J Cardiovasc Prev Rehabil. 2006;13:87–90. https://doi.org/10.1097/00149831-200602000-00013.
Lang J, Scherzer R, Weekley CC, Tien PC, Grunfeld C, Shlipak MG. Serum albumin and short-term risk for mortality and cardiovascular disease among hiv-infected veterans. Aids. 2013;27:1339–43. https://doi.org/10.1097/QAD.0b013e32835f1dd6.
Xu W-H, Dong C, Rundek T, Elkind MS, Sacco RL. Serum albumin levels are associated with cardioembolic and cryptogenic ischemic strokes: Northern manhattan study. Stroke; a J Cereb circulation. 2014;45:973–8.
Ancion A, Allepaerts S, Robinet S, Oury C, Pierard LA, Lancellotti P. Serum albumin level and long-term outcome in acute heart failure. Acta Cardiologica. 2019;74:465–71. https://doi.org/10.1080/00015385.2018.1521557.
Prenner SB, Kumar A, Zhao L, Cvijic ME, Basso M, Spires T, et al. Effect of serum albumin levels in patients with heart failure with preserved ejection fraction (from the topcat trial). Am J Cardiol. 2020;125:575–82. https://doi.org/10.1016/j.amjcard.2019.11.006.
Arques S. Serum albumin and cardiovascular disease: State-of-the-art review. Annales de Cardiologie et d’Angéiologie. Elsevier; 2020, p. 192-200.
Li X, Zhang Y, He Y, Li KX, Xu RN, Wang H, et al. J-shaped association between serum albumin levels and long-term mortality of cardiovascular disease: Experience in national health and nutrition examination survey (2011-2014). Front Cardiovasc Med. 2022;9:1073120 https://doi.org/10.3389/fcvm.2022.1073120.
Zhao D, Jiao H, Zhong X, Wang W, Li L. The association between serum albumin levels and related metabolic factors and atrial fibrillation: A retrospective study. Medicine. 2022;101:e31581. https://doi.org/10.1097/md.0000000000031581.
Bose T, Voruganti VS, Tejero ME, Proffitt JM, Cox LA, VandeBerg JL, et al. Quantitative loci regulating plasma levels of gamma glutamyl transferase and albumin and their genetic correlations with cardiovascular risk factors. Exp Biol Med (Maywood). 2009;234:vi:1519–24. https://doi.org/10.3181/0903-rm-115.
Iglesias J, Abernethy VE, Wang Z, Lieberthal W, Koh JS, Levine JS. Albumin is a major serum survival factor for renal tubular cells and macrophages through scavenging of ros. Am J Physiol. 1999;277:F711–22. https://doi.org/10.1152/ajprenal.1999.277.5.F711.
Peters T Jr. Serum albumin. Adv Protein Chem. 1985;37:161–245. https://doi.org/10.1016/s0065-3233(08)60065-0.
Arquès S, Ambrosi P, Gélisse R, Luccioni R, Habib G. Hypoalbuminemia in elderly patients with acute diastolic heart failure. J Am Coll Cardiol. 2003;42:712–6.
Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008;582:1783–7.
Yamamoto M, Adachi H, Enomoto M, Fukami A, Nakamura S, Nohara Y, et al. Lower albumin levels are associated with frailty measures, trace elements, and an inflammation marker in a cross-sectional study in tanushimaru. Environ Health Preventive Med. 2021;26:1–8.
Moroni F, Ammirati E, Rocca MA, Filippi M, Magnoni M, Camici PG. Cardiovascular disease and brain health: Focus on white matter hyperintensities. Int J Cardiol Heart Vasc. 2018;19:63–9. https://doi.org/10.1016/j.ijcha.2018.04.006.
Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38. https://doi.org/10.1016/s1474-4422(13)70124-8.
Stewart CR, Stringer MS, Shi Y, Thrippleton MJ, Wardlaw JM. Associations between white matter hyperintensity burden, cerebral blood flow and transit time in small vessel disease: An updated meta-analysis. Front Neurol. 2021;12:647848. https://doi.org/10.3389/fneur.2021.647848.
Murray KD, Uddin MN, Tivarus ME, Sahin B, Wang HZ, Singh MV, et al. Increased risk for cerebral small vessel disease is associated with quantitative susceptibility mapping in hiv infected and uninfected individuals. Neuroimage Clin. 2021;32:102786. https://doi.org/10.1016/j.nicl.2021.102786.
Haddow LJ, Sudre CH, Sokolska M, Gilson RC, Williams IG, Golay X, et al. Magnetic resonance imaging of cerebral small vessel disease in men living with hiv and hiv-negative men aged 50 and above. AIDS Res Hum Retroviruses. 2019;35:453–60. https://doi.org/10.1089/aid.2018.0249.
Lewis JP, Suchy-Dicey AM, Noonan C, Blue Bird Jernigan V, Umans JG, Domoto-Reilly K, et al. Associations of binge drinking with vascular brain injury and atrophy in older american indians: The strong heart study. J aging health. 2021;33:51S–9S.
Schutte R, Smith L, Wannamethee G. Alcohol – the myth of cardiovascular protection. Clin Nutr. 2022;41:348–55. https://doi.org/10.1016/j.clnu.2021.12.009.
Watson C, Busovaca E, Foley JM, Allen IE, Schwarz CG, Jahanshad N, et al. White matter hyperintensities correlate to cognition and fiber tract integrity in older adults with hiv. J Neurovirol. 2017;23:422–9. https://doi.org/10.1007/s13365-016-0509-5.
Seider TR, Gongvatana A, Woods AJ, Chen H, Porges EC, Cummings T, et al. Age exacerbates hiv-associated white matter abnormalities. J neurovirology. 2016;22:201–12. https://doi.org/10.1007/s13365-015-0386-3.
Samboju V, Cobigo Y, Paul R, Naasan G, Hillis M, Tsuei T, et al. Cerebrovascular disease correlates with longitudinal brain atrophy in virally suppressed older people living with hiv. J acquired immune Defic syndromes. 2021;87:1079–85. https://doi.org/10.1097/qai.0000000000002683.
Pfefferbaum A, Zhao Q, Pohl KM, Sassoon SA, Zahr NM, Sullivan EV Age-accelerated increase of white matter hyperintensity volumes is exacerbated by heavy alcohol use in people living with hiv. Biological psychiatry 2023. https://doi.org/10.1016/j.biopsych.2023.07.023.
Wada M, Nagasawa H, Kurita K, Koyama S, Arawaka S, Kawanami T, et al. Microalbuminuria is a risk factor for cerebral small vessel disease in community-based elderly subjects. J neurological Sci. 2007;255:27–34. https://doi.org/10.1016/j.jns.2007.01.066.
Knopman DS, Mosley TH Jr., Bailey KR, Jack CR Jr., Schwartz GL, Turner ST. Associations of microalbuminuria with brain atrophy and white matter hyperintensities in hypertensive sibships. J neurological Sci. 2008;271:53–60. https://doi.org/10.1016/j.jns.2008.03.009.
Umemura T, Kawamura T, Sakakibara T, Mashita S, Hotta N, Sobue G. Microalbuminuria is independently associated with deep or infratentorial brain microbleeds in hypertensive adults. Am J Hypertens. 2012;25:430–6. https://doi.org/10.1038/ajh.2011.254.
Vilar-Bergua A, Riba-Llena I, Ramos N, Mundet X, Espinel E, López-Rueda A, et al. Microalbuminuria and the combination of mri markers of cerebral small vessel disease. Cerebrovasc Dis. 2016;42:66–72. https://doi.org/10.1159/000445168.
Cho EB, Shin HY, Park SE, Chun P, Jang HR, Yang JJ, et al. Albuminuria, cerebrovascular disease and cortical atrophy: Among cognitively normal elderly individuals. Sci Rep. 2016;6:20692. https://doi.org/10.1038/srep20692.
Sedaghat S, Ding J, Eiriksdottir G, van Buchem MA, Sigurdsson S, Ikram MA, et al. The ages-reykjavik study suggests that change in kidney measures is associated with subclinical brain pathology in older community-dwelling persons. Kidney Int. 2018;94:608–15. https://doi.org/10.1016/j.kint.2018.04.022.
Yamasaki K, Hata J, Furuta Y, Hirabayashi N, Ohara T, Yoshida D, et al. Association of albuminuria with white matter hyperintensities volume on brain magnetic resonance imaging in elderly japanese- the hisayama study. Circ J. 2020;84:935–42. https://doi.org/10.1253/circj.CJ-19-1069.
Elyas S, Adingupu D, Aizawa K, Casanova F, Gooding K, Fulford J, et al. Cerebral small vessel disease, systemic vascular characteristics and potential therapeutic targets. Aging (Albany NY). 2021;13:22030–9. https://doi.org/10.18632/aging.203557.
Tanaka K, Miwa K, Takagi M, Sasaki M, Yakushiji Y, Kudo K, et al. Increased cerebral small vessel disease burden with renal dysfunction and albuminuria in patients taking antithrombotic agents: The bleeding with antithrombotic therapy 2. J Am Heart Assoc. 2022;11:e024749. https://doi.org/10.1161/jaha.121.024749.
Zuccalà G, Marzetti E, Cesari M, Monaco MRL, Antonica L, Cocchi A, et al. Correlates of cognitive impairment among patients with heart failure: Results of a multicenter survey. Am J Med. 2005;118:496–502.
Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P. Serum inflammatory proteins and cognitive decline in older persons. Neurology. 2005;64:1371–7. https://doi.org/10.1212/01.Wnl.0000158281.08946.68.
Ng TP, Feng L, Niti M, Yap KB. Albumin, haemoglobin, bmi and cognitive performance in older adults. Age Ageing. 2008;37:423–9. https://doi.org/10.1093/ageing/afn102.
Mizrahi EH, Blumstein T, Arad M, Adunsky A. Serum albumin levels predict cognitive impairment in elderly hip fracture patients. Am J Alzheimer’s Dis Other Dement®. 2008;23:85–90.
Llewellyn DJ, Langa KM, Friedland RP, Lang IA. Serum albumin concentration and cognitive impairment. Curr Alzheimer Res. 2010;7:91–6.
Shen J, Amari N, Zack R, Skrinak RT, Unger TL, Posavi M, et al. Plasma mia, crp, and albumin predict cognitive decline in parkinson’s disease. Ann Neurol. 2022;92:255–69. https://doi.org/10.1002/ana.26410.
Seo M, Watanabe T, Yamada T, Morita T, Kawasaki M, Kikuchi A, et al. The clinical relevance of mild cognitive impairment in acute heart failure: A comparison with cognitive impairment. J Cardiol 2023. https://doi.org/10.1016/j.jjcc.2023.08.017.
Shaharir SS, Osman SS, Md Rani SA, Sakthiswary R, Said MSM. Factors associated with increased white matter hyperintense lesion (wmhi) load in patients with systemic lupus erythematosus (sle). Lupus. 2018;27:25–32. https://doi.org/10.1177/0961203317707062.
Kim JW, Byun MS, Lee JH, Yi D, Jeon SY, Sohn BK, et al. Serum albumin and beta-amyloid deposition in the human brain. Neurology. 2020;95:e815–e26. https://doi.org/10.1212/wnl.0000000000010005.
Pfefferbaum A, Zahr NM, Sassoon SA, Kwon D, Pohl KM, Sullivan EV. Accelerated and premature aging characterizing regional cortical volume loss in human immunodeficiency virus infection: Contributions from alcohol, substance use, and hepatitis c coinfection. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:844–59. https://doi.org/10.1016/j.bpsc.2018.06.006.
Sullivan EV, Zahr NM, Sassoon SA, Thompson WK, Kwon D, Pohl KM, et al. The role of aging, drug dependence, and hepatitis c comorbidity in alcoholism cortical compromise. JAMA psychiatry 2018. https://doi.org/10.1001/jamapsychiatry.2018.0021.
First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for dsm-iv axis i disorders (scid) version 2.0. New York, NY.: Biometrics Research Department, New York State Psychiatric Institute; 1998.
Skinner HA, Sheu WJ. Reliability of alcohol use indices. The lifetime drinking history and the mast. J Stud alcohol. 1982;43:1157–70.
Hollingshead A. Four-factor index of social status. New Haven, CT: Department of Sociology, Yale University; 1975.
Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the alcohol use disorders identification test (audit). Alcohol, Clin Exp Res. 1997;21:613–9.
Lundin A, Hallgren M, Balliu N, Forsell Y. The use of alcohol use disorders identification test (audit) in detecting alcohol use disorder and risk drinking in the general population: Validation of audit using schedules for clinical assessment in neuropsychiatry. Alcohol, Clin Exp Res. 2015;39:158–65. https://doi.org/10.1111/acer.12593.
Babor T, Biddle-Higgins J, Saunders J, Monteiro M. Audit: The alcohol use disorders identification test: Guidelines for use in primary health care. Geneva, Switzerland: World Health Organization; 2001.
Zahr NM, Pohl KM, Kwong AJ, Sullivan EV, Pfefferbaum A Preliminary evidence for a relationship between elevated plasma tnfα and smaller subcortical white matter volume in hcv infection irrespective of hiv or aud comorbidity. International Journal of Molecular Sciences 2021;22. https://doi.org/10.3390/ijms22094953.
Kwong AJ, Zahr NM. Serum biomarkers of liver fibrosis identify globus pallidus vulnerability. NeuroImage: Clin. 2023;37:103333. https://doi.org/10.1016/j.nicl.2023.103333.
Coupe P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C. An optimized blockwise nonlocal means denoising filter for 3-d magnetic resonance images. IEEE Trans Med Imaging. 2008;27:425–41. https://doi.org/10.1109/TMI.2007.906087.
Smith SM. Fast robust automated brain extraction. Hum brain Mapp. 2002;17:143–55. https://doi.org/10.1002/hbm.10062.
Cox RW. Afni: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73.
Iglesias JE, Liu CY, Thompson PM, Tu Z. Robust brain extraction across datasets and comparison with publicly available methods. IEEE Trans Med Imaging. 2011;30:1617–34. https://doi.org/10.1109/TMI.2011.2138152.
Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ants similarity metric performance in brain image registration. NeuroImage. 2011;54:2033–44. https://doi.org/10.1016/j.neuroimage.2010.09.025.
Sadananthan SA, Zheng W, Chee MW, Zagorodnov V. Skull stripping using graph cuts. NeuroImage. 2010;49:225–39. https://doi.org/10.1016/j.neuroimage.2009.08.050.
Rohlfing T, Brandt R, Menzel R, Maurer CR Jr. Evaluation of atlas selection strategies for atlas-based image segmentation with application to confocal microscopy images of bee brains. NeuroImage. 2004;21:1428–42.
Jiang J, Paradise M, Liu T, Armstrong NJ, Zhu W, Kochan NA, et al. The association of regional white matter lesions with cognition in a community-based cohort of older individuals. Neuroimage Clin. 2018;19:14–21. https://doi.org/10.1016/j.nicl.2018.03.035.
Quest Diagnostics. Albumin test detail. In: Diagnostics Q, editor. online. online: Quest Diagnostics; 2019.
Zahr NM. Peripheral tnfalpha elevations in abstinent alcoholics are associated with hepatitis c infection. PLoS One. 2018;13:e0191586. https://doi.org/10.1371/journal.pone.0191586.
Piekarski D, Sullivan EV, Pfefferbaum A, Zahr NM. Poor subjective sleep predicts compromised quality of life but not cognitive impairment in abstinent individuals with alcohol use disorder. Alcohol. 2022;103:37–43. https://doi.org/10.1016/j.alcohol.2022.07.001.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol). 1995;57:289–300.
SAS Institute Inc. The varclus procedure. In: Inc. SI, editor. SAS/STAT 14.3 User’s Guide. Cary, NC: 2017.
Shu L, Zhong K, Chen N, Gu W, Shang W, Liang J, et al. Predicting the severity of white matter lesions among patients with cerebrovascular risk factors based on retinal images and clinical laboratory data: A deep learning study. Front Neurol. 2023;14:1168836. https://doi.org/10.3389/fneur.2023.1168836.
Gronewold J, Jokisch M, Schramm S, Himpfen H, Ginster T, Tenhagen I, et al. Periventricular rather than deep white matter hyperintensities mediate effects of hypertension on cognitive performance in the population-based 1000brains study. J Hypertens. 2022;40:2413–22. https://doi.org/10.1097/hjh.0000000000003270.
Phuah CL, Chen Y, Strain JF, Yechoor N, Laurido-Soto OJ, Ances BM, et al. Association of data-driven white matter hyperintensity spatial signatures with distinct cerebral small vessel disease etiologies. Neurology. 2022;99:e2535–e47. https://doi.org/10.1212/wnl.0000000000201186.
Wang M, Feng H, Zhang S, Luo Z, Liang Y, Xu Y, et al. Association between red blood cell distribution width and white matter hyperintensities: A large-scale cross-sectional study. Brain Behav. 2020;10:e01739. https://doi.org/10.1002/brb3.1739.
Keller JA, Kant IMJ, Slooter AJC, van Montfort SJT, van Buchem MA, van Osch MJP, et al. Different cardiovascular risk factors are related to distinct white matter hyperintensity mri phenotypes in older adults. Neuroimage Clin. 2022;35:103131. https://doi.org/10.1016/j.nicl.2022.103131.
ten Dam VH, van den Heuvel DM, de Craen AJ, Bollen EL, Murray HM, Westendorp RG, et al. Decline in total cerebral blood flow is linked with increase in periventricular but not deep white matter hyperintensities. Radiology. 2007;243:198–203. https://doi.org/10.1148/radiol.2431052111.
Lam V, Albrecht MA, Takechi R, Heidari-Nejad S, Foster JK, Mamo JC. Neuropsychological performance is positively associated with plasma albumin in healthy adults. Neuropsychobiology. 2014;69:31–8. https://doi.org/10.1159/000356967.
Wei H, Liu D, Geng L, Liu Y, Wang H, Yan F. Application value of serum metabolic markers for cognitive prediction in elderly epilepsy. Neuropsychiatr Dis Treat. 2022;18:2133–40. https://doi.org/10.2147/ndt.S371751.
Ma ZY, Wu YY, Cui HY, Yao GY, Bian H. Factors influencing post-stroke cognitive impairment in patients with type 2 diabetes mellitus. Clin Inter Aging. 2022;17:653–64. https://doi.org/10.2147/cia.S355242.
Iwasaki M, Motokawa K, Watanabe Y, Hayakawa M, Mikami Y, Shirobe M, et al. Nutritional status and body composition in cognitively impaired older persons living alone: The takashimadaira study. PLoS One. 2021;16:e0260412. https://doi.org/10.1371/journal.pone.0260412.
Wang L, Wang F, Liu J, Zhang Q, Lei P. Inverse relationship between baseline serum albumin levels and risk of mild cognitive impairment in elderly: A seven-year retrospective cohort study. Tohoku J Exp Med. 2018;246:51–7. https://doi.org/10.1620/tjem.246.51.
Zhu JJ, Chen YJ, Chen LL, Zhao LJ, Zhou P. Factors that contribute to the cognitive impairment in elderly dialysis patients. Ther Apher Dial. 2022;26:632–9. https://doi.org/10.1111/1744-9987.13740.
Pei X, Lai S, He X, Masembe NP, Yuan H, Yong Z, et al. Mild cognitive impairment in maintenance hemodialysis patients: A cross-sectional survey and cohort study. Clin Inter Aging. 2019;14:27–32. https://doi.org/10.2147/cia.S178854.
Elliott C, Frith J, Day CP, Jones DE, Newton JL. Functional impairment in alcoholic liver disease and non-alcoholic fatty liver disease is significant and persists over 3 years of follow-up. Digestive Dis Sci. 2013;58:2383–91. https://doi.org/10.1007/s10620-013-2657-2.
Zhang YH, Yang ZK, Wang JW, Xiong ZY, Liao JL, Hao L, et al. Cognitive changes in peritoneal dialysis patients: A multicenter prospective cohort study. Am J Kidney Dis. 2018;72:691–700. https://doi.org/10.1053/j.ajkd.2018.04.020.
Heaton RK, Franklin DR Jr, Deutsch R, Letendre S, Ellis RJ, Casaletto K, et al. Neurocognitive change in the era of hiv combination antiretroviral therapy: The longitudinal charter study. Clin Infect Dis. 2015;60:473–80.
Barokar J, McCutchan A, Deutsch R, Tang B, Cherner M, Bharti AR. Neurocognitive impairment is worse in hiv/hcv-coinfected individuals with liver dysfunction. J neurovirology. 2019;25:792–9. https://doi.org/10.1007/s13365-019-00767-6.
Parsons TD, Tucker KA, Hall CD, Robertson WT, Eron JJ, Fried MW, et al. Neurocognitive functioning and haart in hiv and hepatitis c virus co-infection. AIDS. 2006;20:1591–5. https://doi.org/10.1097/01.aids.0000238404.16121.47.
Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Borenstein J. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol. 2002;39:1780–6. https://doi.org/10.1016/S0735-1097(02)01854-5.
Frisbie JH. Anemia and hypoalbuminemia of chronic spinal cord injury: prevalence and prognostic significance. Spinal Cord. 2010;48:566–9. https://doi.org/10.1038/sc.2009.163.
Gillum RF. The association between serum albumin and hdl and total cholesterol. J Natl Med Assoc. 1993;85:290–2.
Haybar H, Pezeshki SMS, Saki N. Evaluation of complete blood count parameters in cardiovascular diseases: An early indicator of prognosis? Exp Mol Pathol. 2019;110:104267. https://doi.org/10.1016/j.yexmp.2019.104267.
Li B, Du B, Gu Z, Wu C, Tan Y, Song C, et al. Correlations among peripheral blood markers, white matter hyperintensity, and cognitive function in patients with non-disabling ischemic cerebrovascular events. Front Aging Neurosci. 2022;14. https://doi.org/10.3389/fnagi.2022.1023195.
Godsland IF, North BV, Johnston DG. Simple indices of inflammation as predictors of death from cancer or cardiovascular disease in a prospective cohort after two decades of follow-up. Qjm. 2011;104:387–94. https://doi.org/10.1093/qjmed/hcq213.
Seo IH, Lee YJ. Usefulness of complete blood count (CBC) to assess cardiovascular and metabolic diseases in clinical settings: a comprehensive literature review. Biomedicines 2022;10. https://doi.org/10.3390/biomedicines10112697.
Anavekar NS, Gans DJ, Berl T, Rohde RD, Cooper W, Bhaumik A, et al. Predictors of cardiovascular events in patients with type 2 diabetic nephropathy and hypertension: a case for albuminuria. Kidney Int Suppl. 2004:S50-5. https://doi.org/10.1111/j.1523-1755.2004.09213.x.
Wang Z, Hoy WE, Wang Z. The correlates of urinary albumin to creatinine ratio (acr) in a high risk Australian aboriginal community. BMC Nephrol. 2013;14:176.
Boorsma EM, ter Maaten JM, Damman K, van Essen BJ, Zannad F, van Veldhuisen DJ, et al. Albuminuria as a marker of systemic congestion in patients with heart failure. Eur Heart J. 2022;44:368–80. https://doi.org/10.1093/eurheartj/ehac528.
Nour M, Hegazy A, Mosbah A, Abdelaziz A, Fawzy M. Role of microalbuminuria and hypoalbuminemia as outcome predictors in critically ill patients. Crit Care Res Pr. 2021;2021:6670642. https://doi.org/10.1155/2021/6670642.
Caironi P, Gattinoni L. The clinical use of albumin: the point of view of a specialist in intensive care. Blood Transfus. 2009;7:259–67. https://doi.org/10.2450/2009.0002-09.
Choi JW, Park JS, Lee CH. Genetically determined hypoalbuminemia as a risk factor for hypertension: Instrumental variable analysis. Sci Rep. 2021;11:11290. https://doi.org/10.1038/s41598-021-89775-3.
Unger JK, Horn NA, Kashefi A, Blumberg A, Klosterhalfen B, Rossaint R. The influence of hypoalbuminemia on maximal flow rates and transmembrane pressure during plasmapheresis-an in vitro study. Blood Purif. 2001;19:408–16. https://doi.org/10.1159/000046972.
Weisberg HF. Osmotic pressure of the serum proteins. Ann Clin Lab Sci. 1978;8:155–64.
Funding
This study was supported with grant funding from the National Institute of Alcohol Abuse and Alcoholism (NIAAA) including U01 AA017347, R01 AA005965, and R01 AA010723.
Author information
Authors and Affiliations
Contributions
Zahr: conceptualization, methodology, validation, formal analysis, resources, data curation, writing—original draft, writing—review and editing, supervision, project administration, funding acquisition. Pfefferbaum: validation, formal analysis, resources, data curation, writing - review and editing, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Review Board of Stanford University and SRI International.
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zahr, N.M., Pfefferbaum, A. Serum albumin and white matter hyperintensities. Transl Psychiatry 14, 233 (2024). https://doi.org/10.1038/s41398-024-02953-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-024-02953-5
- Springer Nature Limited