Abstract
Objective
To determine the effect of parental socioeconomic status (SES) on the postnatal growth of preterm infants.
Methods
A systematic review (PROSPERO registration CRD42020225714) of original articles from Medline, Embase, CINAHL Plus and Web of Science published 1946-2023 was undertaken. Studies were included if they reported anthropometric growth outcomes for preterm infants according to parental SES. Data extraction and assessments of bias and health equity impact were conducted using custom-designed forms.
Results
A narrative synthesis of twelve included studies was performed. Most infants were moderate to late preterm. The settings, growth outcomes, timings of growth measurement, and SES measures were heterogenous. Six studies demonstrated an adverse effect of low parental SES on the extrauterine growth of preterm infants, five studies showed no effect, and one study showed a potentially beneficial effect. All studies had a high risk of bias, especially confounding and selection bias. The health equity impact of included studies was largely negative.
Conclusion
Limited and low-quality evidence suggests that socioeconomic minoritisation may adversely impact the growth of preterm infants, thereby widening existing socioeconomic health inequities. Observational studies informed by theorisation of the mechanistic pathways linking socioeconomic minoritisation to adverse postnatal growth are required to identify targets for intervention.
Impact
-
Limited evidence suggests low parental socioeconomic status (SES) adversely affects the postnatal growth of preterm infants across different settings.
-
Early growth of preterm infants predicts neurodevelopmental outcomes and the risk of cardiovascular and metabolic disease in adulthood.
-
Systematic screening of over 15,000 articles identified only twelve studies which reported postnatal growth outcomes for preterm infants according to parental SES.
-
The health equity impact of the included studies was systematically assessed, and found to be negative overall.
-
This study highlights limitations in existing evidence on the association between parental SES and postnatal growth, and delineates avenues for future research.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Socioeconomic deprivation has adverse health consequences throughout the life course. It is associated with adverse pregnancy outcomes, greater infant and childhood mortality, poorer development in childhood, a shorter life expectancy and poorer health-related quality of life1,2,3,4.
The deleterious impact of socioeconomic deprivation on maternal and child health outcomes in the perinatal period can lead to the intergenerational transfer of morbidity, perpetuating health disparities. Low socioeconomic status (SES) is a risk factor for preterm birth1,4 and intrauterine growth restriction5 across the world. Thus, by the time of birth, socioeconomic minoritisation is already embodied in infants due to poorer growth in-utero and prematurity.
The extrauterine growth of preterm infants has been extensively investigated, as it has implications for both childhood development and risk of adult disease. Early postnatal growth in preterm infants is an important predictor of later neurodevelopment outcomes6,7. On the other hand, accelerated growth following preterm birth is associated with later cardiovascular and metabolic disease8,9. The postnatal weight loss consistently observed in previous studies of preterm infants10,11 has been shown to be avoidable with the standardised implementation of improved neonatal guidelines12.
Eliciting the impact of social circumstances on outcomes of preterm infants has been identified as a key priority for collaborative research13. Considering the significant impact of preterm infants’ postnatal growth pattern on their long-term health, and the fact that preterm birth occurs disproportionately in those who are socioeconomically deprived, the effect of SES on the extrauterine growth of preterm infants needs to be delineated. We conducted a systematic literature review to investigate the impact of parental SES on postnatal growth outcomes of preterm infants.
Methods
This study adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (refer to the PRISMA Checklist). Search terms, eligibility criteria, and proposed data synthesis methods were registered with PROSPERO (CRD42020225714) prior to the start of the review. We included original research studies reporting data from humans, including clinical trials and observational studies, which included preterm infants born at less than 37 weeks’ gestational age. We only included studies which reported a measure of parental SES as an exposure and reported anthropometric infant growth outcomes according to parental SES. The eligibility criteria are summarised in Table S1. The initial inclusion criteria specified English language articles published between 1990 and 2020, but the inclusion criteria were later amended to original research studies in all languages published between 1946 and 2023, including observational studies and clinical trials. The reference lists of relevant reviews were searched to identify additional eligible articles.
Searches and information sources
A literature search was conducted using the search strategy detailed in Table S2. The electronic databases MEDLINE (1946 onwards) and Embase (1947 onwards), CINAHL Complete and the Web of Science website (wok.mimas.ac.uk, 1970 onwards) were searched for published literature and conference proceedings. No date or language restrictions were applied. Due to the lack of availability of translators, Google Translate (translate.google.com) was used for non-English language articles. The last search was run on 04 February 2023.
Study selection
Titles and abstracts were manually screened, and full texts were screened where the article’s eligibility for inclusion remained unclear. No automation tools were used in the article selection process. The full text of any potentially eligible article was then reassessed against the selection criteria and included or excluded as appropriate. Two authors (K.R. and A.Y.) independently screened a subset 1037 titles and abstracts; conflicts were resolved through discussion to ensure consistent application of the eligibility criteria. A single author (K.R.) conducted the remainder of title and abstract screening, and full text screening.
Data collection
Data extraction was conducted by a single author (K.R.) using a purpose-built data collection form (Table S3).
Assessment of risk of bias
Risk of bias within studies was conducted by a single author (K.R.) by assessing the study design and methodology using a custom-designed table, adapted from the CASP checklists14 and the framework developed by Viswanathan et al.15 for observational studies (Table S4). Tools designed to assess the risk of bias for Randomised Controlled Trials (RCTs) were not used as no RCTs were included.
Data synthesis
Growth outcomes for preterm infants according to parental SES were extracted and assessed for suitability for meta-analysis.
Results
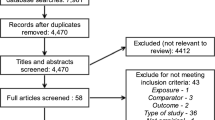
Twelve out of 15,219 articles assessed were included (Fig. 1): Ahn16, Bocca-Tjeertes17, Sammy18, Teranishi19, Holmqvist20, Ghods21, Ni22, Kelleher23, Fu24, Liang25, Sices26 and Peterson27. Seventeen articles reported some measure of parental SES and growth outcomes for preterm infants but were excluded because growth data were not reported separately according to parental SES (e.g. only the regression or correlation coefficient or a test statistic was reported, or the authors mentioned a lack of association between parental SES and growth outcomes without presenting data). A summary of these articles is presented in Table S5. The authors of these studies were not contacted to provide raw data.
Characteristics of included studies are summarised in Table 1. Seven of the included studies16,18,19,20,22,23,24analysed data collected prospectively for a cohort of children, whereas three studies21,25,26 analysed retrospectively collected data, one study17analysed a mixture of retrospectively collected and cross-sectional data for the same cohort of children, and one study27analysed data collected prospectively and retrospectively from cohorts. Nine studies were conducted in high-income countries (South Korea, United States, United Kingdom, the Netherlands, Austria and Sweden), two studies in an upper middle-income country (China), and one study in a lower middle-income country (Kenya). Six studies had mainly moderate to late preterm infants16,17,18,20,23,24, whereas five studies had mainly very and extremely preterm infants21,22,25,26,27. Most infants did not have congenital anomalies or syndromes.
Due to the heterogeneity of growth and parental SES measures across studies, we were unable to perform a meta-analysis and instead conducted a narrative synthesis of the findings28. Ten studies considered maternal or primary caregiver education a measure of parental SES17,18,20,21,22,23,24,25,26,27. Three studies considered family income17,23,25. Three studies used parental occupational class as a proxy for SES19,22,24. In the study by Ghods et al., a psychologist interviewing the parents also rated families’ Home Facilities, and estimated a composite Financial Situation measure. Interestingly, Ahn et al. considered maternal employment to be a sociocultural factor due to insufficient support for maternity leave and childcare for employed mothers (Table 1).
Study findings
Seven studies demonstrated an effect of SES on the postnatal growth of preterm infants. Findings are summarised in Table 2.
Ahn et al. showed that the weight gain of infants born to employed mothers was slower than that of infants born to unemployed mothers from birth to 6 months (p < 0.001). Bocca-Tjeertes et al. showed that a low level of maternal education, but not low family income, was associated with restricted head circumference growth at 1 year compared to the population average. Teranishi et al. showed that at all time points (7, 11, 16 and 23 years), the height deficit between low birthweight (LBW, < 2500 g) and appropriate birthweight (ABW, ≥ 2500 g) preterm infants in the higher social classes was lower when compared with the height deficit between LBW and ABW preterm infants in the lower social classes.
Ghods et al. demonstrated that HC catch-up was significantly positively associated with a longer duration of maternal education (p = 0.012), and also with the families’ Home Facilities (p = 0.027) and Financial ratings (p = 0.001). Liang et al. showed that infants from households with the lowest monthly income ( < 3000 CNY) had the lowest weight, body length and head circumference measurements at 12 months.
Ni et al. reported that parental occupational SES was a moderator of the relationship between birthweight and BMI at 19 years of age in preterm-born participants: an inverse association was observed between birthweight z-score and BMI at 19 years in participants with lower SES and no association was observed among participants with higher SES.
Kelleher et al. reported that maternal education, but not annual family income, was associated with the incidence of failure to thrive (FTT) up to 36 months corrected age. Interestingly, the multivariable regression showed that infants of mothers with a college degree or higher education were at increased risk of FTT compared to infants of mothers with college education without a degree (reference group), after adjusting for demographic and birth variables.
Five studies did not show an association between parental SES and the postnatal growth of preterm infants. Sammy et al. demonstrated no statistically significant difference in the odds of growth deficit at 2 weeks post-discharge from the newborn unit between infants born to mothers with primary, secondary and tertiary level education compared to mothers with no education. Holmqvist et al. showed no significant differences in the weekly growth increments in weight, length or head circumference of infants of well educated mothers compared with infants of less well educated mothers. Fu et al. Sices et al. and Peterson et al. showed that there was no significant association between maternal educational level and the prevalence of overweight/obesity at 4 to 7 years corrected age; the incidence of growth failure up to 20 months; and the prevalence of subnormal head circumference at school age respectively.
Risk of bias
The risk of bias within studies is summarised in Table 3.
The reported associations (or lack thereof) between parental SES and growth outcomes in all twelve studies have a high risk of confounding bias. Ten studies did not report differences in baseline infant and parental characteristics for groups with differing parental SES; of the studies that did, neither adjusted for the detected differences in their analyses.
In their analysis of growth outcomes according to parental SES, only three studies17,23,25adjusted for birth characteristics and/or comorbidities. Nine studies16,18,19,20,21,22,24,26,27did not adjust for the variables shown by their own analyses to be significantly associated with the growth outcome(s) under consideration. The two studies which reported BMI or overweight/obesity as a growth outcome measured but did not adjust for postnatal growth trajectories in their analysis of the association between parental SES and BMI or overweight/obesity22,24.
Six of the twelve studies reported missing data16,17,18,19,21,26. The numbers of infants for whom growth data was available at each time point are not reported by Kelleher et al. or Holmqvist et al., but any missing data will have had a large impact on the analysis in the latter study due to the small sample size.
The risk of detection bias is significant in eight studies. The height at 23 years in Teranishi et al’s study was self-reported – self-reported height has been shown to overestimate actual height29. The primary growth outcomes reported in Sammy et al.’s study, “optimal growth” and “growth deficit”, were undefined. Furthermore, as the timing of follow-up in this study was not standardised, infants could have been at different points in their growth trajectories at follow-up – previous studies have demonstrated that the growth trajectories of preterm infants vary during the first few days to weeks of life6,10,11,12. Holmvist et al. used Student’s t-tests to compare weekly growth between infants of well-educated and less well-educated mothers within discrete time periods; they did not use statistical methods which allow comparison of growth trends over the entire study period (birth to 48 months) whilst accounting for correlation between repeated measures. The studies by Sammy et al. and by Holmqvist et al. are likely to be underpowered due to the small samples of infants born to mothers with no education (n = 7) and infants born to well-educated mothers (n = 8) respectively. Ghods et al. used two interviewer-dependent measures of parental SES in their analyses; it is not specified whether the interviews were administered in German or a language of the parents’ choice, despite 40% of infants being from immigrant families.
In the studies by Ni et al. and Fu et al. BMI – or childhood overweight/obesity as defined by BMI z-scores – were the only growth outcomes reported according to parental SES. The use of BMI for the measurement of body fat mass has been widely criticised30. Furthermore, the exclusive reporting of BMI did not offer any information about height or weight growth.
The risk of selection bias is significant in eleven studies. We considered all studies which were likely to disproportionately exclude socioeconomically minoritised participants to be at moderate or high risk of selection bias, even if the study design did not specify selecting participants based on parental SES. We discuss this in detail in the next section.
Health equity impact assessment
Given the subject matter of this review, we considered the health equity impact of the included studies using the framework developed by Castillo and Harris31,32,33,34, although this was not specified in our registered protocol (Table 4). Prompted by the disproportionate impact of the COVID-19 pandemic on minoritised communities, this framework was developed by researchers with expertise in health inequities and community-partnered research to facilitate consideration of studies’ health equity impact during peer review. The focus areas in the impact assessment are based on published guidance on conducting and evaluating health inequity and community-based participatory research.
Socioeconomically minoritised infants were underrepresented in eleven studies. Bocca-Tjeertes et al reported that the mothers in the non-response group were more likely to have a lower level of education. Several studies specified eligibility criteria which disproportionately excluded socioeconomically minoritised participants16,20,23,25. Finally, the studies which excluded infants lost to follow-up19,21 or infants without growth measurements at specific time points22,24,26,27 disadvantaged participants from disinvested communities35,36,37.
We also considered the impact of the study’s setting on representativeness. Ahn et al actively excluded infants born outside an urban University hospital, and Sices et al, Ghods et al, Holmqvist et al, Peterson et al and Liang et al conducted their follow-up at urban tertiary or teaching hospitals. This would have disproportionately affected the inclusion and follow-up attendance of participants from rural communities38,39. Conversely, the study by Sammy et al had a positive health equity impact as it was based in a District Hospital serving infants in rural Kenya40.
Only one study, by Ahn et al., had a positive health equity impact with regard to the contextualisation and interpretation of data. Both Sices et al. and Peterson et al used a composite “social” or “sociodemographic” risk score, but did not justify the use of these scores. Bocca-Tjeertes et al. invoked biological essentialism by proposing a genetic mechanism for the reported association between low maternal education and head circumference growth restraint. The studies by Sammy et al., Teranishi et al., Holmqvist et al. and Ghods et al. acknowledged the role of socioeconomic or sociocultural factors, but did not propose mechanistic pathways by which resultant minoritisation shapes exposure to structural determinants of health. Similarly, Ni et al. did not consider the structural determinants of pre-pregnancy weight that may mediate the relationship between lower parental socioeconomic status, birthweight, and BMI of preterm-born infants at 19 years.
Four studies23,24,26,27 did not offer an explanation for the reported lack of association between parental SES and growth outcomes. Whereas, Liang et al. and Ghods et al. did not discuss their findings of a relationship between parental SES and measured growth outcomes.
None of the studies reported methods used for community engagement in research participation.
Discussion
This is the first systematic review to directly investigate the role of SES in the postnatal growth of preterm infants. Only twelve out of nearly 15,000 screened articles met the eligibility criteria as most studies did not explicitly report growth outcomes according to parental SES. The settings, growth outcomes, timings of growth outcome measurement, and measures of SES used in these studies were highly heterogeneous.
Six studies16,17,19,21,22,25reported a relationship between lower parental SES and patterns of growth in preterm infants likely to be associated with adverse longer term neurodevelopmental or metabolic outcomes, whereas five studies reported no association between parental SES and postnatal growth18,20,24,26,27. A single study, Kelleher et al., showed that infants of mothers with a college degree or greater education compared to mothers with some college education without a degree were at increased risk of developing failure to thrive23. However, the choice of reference group in the analysis (i.e. mothers with some college education, one of the intermediate categories) did not allow for a robust comparison of growth outcomes according to parental SES as those at the extremes of socioeconomic privilege and deprivation could not be compared.
All included articles have a high risk of bias. Ten studies do not report differences in baseline characteristics of infants born to parents of different SES categories, and only three studies adjust for birth characteristics or postnatal factors affecting the extrauterine growth of preterm infants, such as anthropometric measurements at birth11, and neonatal comorbidities including sepsis, chronic lung disease, postnatal steroid treatment, and necrotising enterocolitis11,41,42. Ni et al. and Fu et al. do not adjust for postnatal growth trajectories when analysing the relationship between parental SES and BMI at 19 years and 4 to 7 years respectively, even though there is evidence to suggest the rate of postnatal “catch up” growth may be associated with fat deposition43. Eleven studies suffer from selection bias as study participants are less likely to have a low SES.
There may also be a risk of residual confounding due to the choice of parental SES measure in the included studies. For example, the granularity of information regarding parental SES is often limited as several studies use binary classifications of maternal education17,20,21,22,26,27. Furthermore, although maternal education and parental occupation have been used widely as measures of SES, household income has been shown to specifically affect children’s cognitive, socio-behavioural and health outcomes44. Only three studies17,23,25 measure household income, and only two of these studies use a quantitative categorisation of income23,25. Notably, in the study by Liang et al., an association was seen between all three growth outcomes (height, weight and head circumference) at 12 months and monthly household income, whereas no association was seen with primary caregiver education. Kelleher et al. also use a three-level classification of family income as a measure of parental SES but do not demonstrate any association with infant growth, whereas there is an association between maternal education and infant growth. The association between income and growth may not have been revealed in the multiple regression analysis due to collinearity between the income and maternal education variables.
Poorer outcomes during neonatal intensive care unit (NICU) admission and a detrimental impact on parents and families may contribute to socioeconomic disparities in the postnatal growth of preterm infants. Despite the protocolised nature of neonatal intensive care, the adverse effects of socioeconomic deprivation are seen in the quality of care. In the United States, NICUs with a greater proportion of patients from a lower socioeconomic background, and units situated in more deprived areas, were more likely to have a lower Baby-MONITOR quality score45. Socioeconomic disparities have been noted in the outcomes of neonatal sepsis, a common diagnosis which often necessitates NICU admission46.
Differences in nutritional practices may also mediate inequities in the postnatal growth of preterm infants. Early nutrient delivery in the NICU has been shown to be associated with growth velocity in the neonatal period47. Breastmilk is known to reduce the risk of neonatal morbidities and improve neurodevelopmental outcomes in preterm infants48,49,50. However, socioeconomically minoritised mothers are less likely to initiate51 and continue breastfeeding preterm infants52.
The burden of unmet material needs among socioeconomically minoritised families is likely to contribute to adverse growth outcomes for preterm infants; in a cohort of low-income families with preterm infants across the US, 26% suffered from food insecurity, 33% from housing insecurity, and 28% from energy insecurity53. These material inequities result in obstacles to, and increase the opportunity cost of, practices such as breastfeeding and attending follow-up appointments.
Pathophysiological pathways may link the experience of stress in parents to poor growth outcomes in preterm-born children. Low SES has been shown to exacerbate the impact of negative life events on antenatal anxiety and depression54. Maternal antenatal stress exposure, in turn, was associated with white matter microstructural changes in preterm infants at term-equivalent age55.
Financial stress related to NICU care may also limit the involvement of parents from historically and contemporaneously excluded communities, and impact attendance at follow-up appointments35. In a prospective cohort study of 169 mother-infant dyads, an annual household income > $100,000 was associated with more time spent in the NICU56. Parents of preterm infants are at higher risk of reducing or stopping work57, particularly if their child has additional medical comorbidities58. Furthermore, parents of preterm infants face substantial travel and subsistence expenses whilst their infants are in the neonatal unit59,60,61.
Accordingly, strategies to reduce the duration of inpatient hospitalisation may be effective in alleviating some of the financial burden faced by parents. Breastfeeding rates could be maintained with adequate nursing support following early discharge of preterm infants from the NICU once full oral feeding had been established62. In preterm infants requiring tube feeding, conventional NICU inpatient care and home tele-healthcare resulted in comparable weight for age z-scores at discharge and exclusive breastfeeding rates63. On the other hand, unidimensional strategies tackling a single cost (e.g. parking charges) are unlikely to be effective64.
Individual and neighbourhood-level socioeconomic deprivation continue to affect the health of preterm infants throughout childhood. Ex-preterm children are at higher risk of hospitalisation for bronchiolitis65and poverty has been shown to be associated with poorer outcomes in critically ill children admitted with bronchiolitis66. Preterm-born children with bronchopulmonary dysplasia living in the most deprived areas in Philadelphia had increased Emergency Department visits, hospital readmissions and activity limitations compared with those in the least deprived areas67. Despite the use of regional follow-up protocols after preterm birth in a setting with well-developed healthcare infrastructure, very preterm-born children living in deprived neighbourhoods in France had more frequent unplanned rehospitalisations68.
Considering the high-intensity clinical support offered in the NICU, there is potential to identify and address individual and systemic barriers in a standardised manner to optimise outcomes for socioeconomically deprived patients. In 2016, the American Academy of Pediatrics recommended universal screening for adverse social determinants of health in paediatric services and onward referral if required69. However, Parker et al. ‘s mixed methods study in two NICUs in “safety-net” hospitals in the US published in 2021 showed that, apart from employment, other unmet basic needs including food insecurity, were not commonly assessed53. The implementation of a standardised screening tool integrating screening and referral into clinical workflow increased systematic screening from 0% to 49%, with 98% of families requesting assistance receiving referrals70. It has been shown that referrals following screening for basic unmet needs at community paediatric outpatient appointments leads to greater receipt of community resources for families71. They also advocate for non-siloed holistic care through screening for postpartum depression and broader screening for parental mental health needs in the NICU72.
The analysis in this review is limited by the fact that we did not examine outcomes by race/ethnicity in the included studies. When considering the communities affected most by socioeconomic minoritisation, it is crucial to consider the impact of structural racism, how it intersects with economic disinvestment, and how it disproportionately exposes adversely racialised people to poverty and its consequences. A well-known manifestation of structural racism is stress. As described by Arline Geronimus73, “weathering” during pregnancy – and throughout the lifecourse –may exacerbate stress-related physiological changes in foetuses55, and worsen the physical and mental health of parents facing combined socioeconomic and racial minoritisation. Furthermore, a systematic review highlighted that black and Hispanic infants in the US were more likely to be cared for in hospitals providing lower quality care with greater neonatal morbidity and mortality74. However, there were also notable within-NICU inequities. In two studies, Black and Hispanic infants were less likely to be referred for follow-up care than white infants. Racial/ethnic inequities were found in NICU breastfeeding rates, with limited breastfeeding education and support listed as contributory factors. Qualitative research with Black birthing people has shown through examples how structural, institutional and interpersonal racism affected the quality of NICU care75. A health system-focused strategy is required to mitigate racial/ethnic inequities in NICU care, including standardised assessments of structural determinants of health, psychosocial support, and support for material needs76.
The studies in this review had a largely negative impact on health equity. In addition to selection bias which disproportionately excluded socioeconomically minoritised participants, those from rural communities may have been impacted by the eligibility criteria and follow-up arrangements for studies based in urban centres16,20,21,25,26,27. This is likely to have differential effects based on local socioeconomic geography. In Korea and China, those in rural areas experience greater socioeconomic minoritisation38, and in the US, those from rural communities are less likely to attend paediatric follow-up appointments39. In western and Nordic European countries, the highest risk of poverty was recorded in people living in cities77. Conversely, Sammy et al.’s study is based in a District Hospital in rural Kenya, where fewer households are in the wealthiest quintile compared to urban areas40.
Theorisation concerning mechanisms by which socioeconomic minoritisation may be affecting postnatal growth is lacking throughout the studies. Only Ahn et al. offer context to explain why the policy and sociocultural environment in Korea systematically disadvantages infants of employed mothers. Opportunities were otherwise missed to identify the processes by which socioeconomic minoritisation actively shapes exposure to structural determinants of health, and to identify targets for intervention and policy change. The genetic link between head circumference growth restraint and low maternal education proposed by Bocca-Tjeertes et al. fails to consider modifiable structural factors; for instance, those experiencing racial/ethnic and socioeconomic minoritisation in the Netherlands have been shown to face barriers accessing specialised health services78. Furthermore, Sices et al. and Peterson et al. use a composite risk score, positioning the minoritisation from a low maternal education level as equivalent to racial/ethnic minoritisation and stigmatisation due to “unmarried” status. The use of these composite scores ignores the pathways by which different structural oppressions may manifest and intersect.
None of the studies report efforts to engage or co-produce research with communities, including the study by Bocca-Tjeertes et al. based in community health centres, or studies which have a very long follow-up period, such as the 23 year follow-up by Teranishi et al. Co-design with community stakeholders may have mitigated loss to follow-up seen disproportionately in socioeconomically minoritised participants35,36,37by allowing researchers to identify and address exclusionary research practices79.
Furthermore, the lived expertise of community members may highlight locally relevant mechanisms by which health inequity due to socioeconomic minoritisation is (re)produced and maintained. In fact, collaboration with community advocates as a means of redistributing power to minoritised communities has been highlighted as a key strategy to avoid harmful health equity “tourism”80. The mechanisms by which lower SES affects preterm infant growth are likely to vary depending on health system infrastructure, political context and intersecting oppressive structures. For example, the cost of accessing healthcare services81may result in catastrophic health expenditure and reduced spending on essential items including food82, thereby affecting infant growth through food insecurity83. Even if healthcare is offered free of cost, preterm birth is associated with the stress of significantly increased economic costs over the first 2 years84, which are likely to be exacerbated with rising costs of living. In India, minoritisation due to caste is associated with higher infant and child mortality rates85. In a study of mothers of preterm infants in Malaysia, Malay, Indian, Chinese and indigenous ethnicities had different positive and negative associations with quality of life in various domains, including physical health, psychological wellbeing and the quality of their environment86.
When co-designing future research, researchers should consider how to capture socioeconomic minoritisation as an exposure, to prevent deficit thinking that avoids consideration of the structural determinants of health87. Variables of interest may include exposure to economic policies (e.g. austerity) associated with greater material impoverishment88, racial-economic segregation resulting from structural racism89, and employment precarity, which is linked to adverse workplace health outcomes90. Given the significance of the intersection between socioeconomic minoritisation and adverse racialisation, future studies investigating the former should also consider measuring structural racism as an exposure91.
Conclusion
The impact of parental SES on the postnatal growth of preterm infants may be a mechanism for the intergenerational transfer of health inequity in socioeconomically minoritised communities. In our systematic review, limited and low certainty evidence suggests that socioeconomic minoritisation may adversely affect the postnatal growth of preterm infants. However, the high risk of bias and lack of adjustment for confounding factors in the included studies compromises the reliability of their findings. Primary observational studies measuring socioeconomic minoritisation, other intersecting oppressions, and antenatal and neonatal factors which are known to impact postnatal growth, will help to identify further targets for intervention and policy change.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Ncube, C. N., Enquobahrie, D. A., Albert, S. M., Herrick, A. L. & Burke, J. G. Association of Neighborhood Context with Offspring Risk of Preterm Birth and Low Birthweight: A Systematic Review and Meta-Analysis of Population-Based Studies. Soc. Sci. Med. 153, 156–164 (2016).
Marmot, M., Allen, J., Boyce, T., Goldblatt, P. & Morrison, J. Health Equity in England: The Marmot Review 10 Years On. (The Health Foundation, 2020).
Karlsson, O., Kim, R., Joe, W. & Subramanian, S. V. Socioeconomic and Gender Inequalities in Neonatal, Postneonatal and Child Mortality in India: A Repeated Cross-Sectional Study, 2005-2016. J. Epidemiol. Community Health 73, 660–667 (2019).
Smith, L. K., Draper, E. S., Manktelow, B. N., Dorling, J. S. & Field, D. J. Socioeconomic Inequalities in Very Preterm Birth Rates. Arch. Dis. Child Fetal Neonatal Ed. 92, F11–F14 (2007).
Villar, J. et al. Preeclampsia, Gestational Hypertension and Intrauterine Growth Restriction, Related or Independent Conditions? Am. J. Obstet. Gynecol. 194, 921–931 (2006).
Franz, A. R. et al. Intrauterine, Early Neonatal, and Postdischarge Growth and Neurodevelopmental Outcome at 5.4 Years in Extremely Preterm Infants after Intensive Neonatal Nutritional Support. Pediatrics 123, e101–e109 (2009).
Ehrenkranz, R. A. et al. Growth in the Neonatal Intensive Care Unit Influences Neurodevelopmental and Growth Outcomes of Extremely Low Birth Weight Infants. Pediatrics 117, 1253–1261 (2006).
Fagerberg, B., Bondjers, L. & Nilsson, P. Low Birth Weight in Combination with Catch-up Growth Predicts the Occurrence of the Metabolic Syndrome in Men at Late Middle Age: The Atherosclerosis and Insulin Resistance Study. J. Intern Med. 256, 254–259 (2004).
Lapillonne, A. & Griffin, I. J. Feeding Preterm Infants Today for Later Metabolic and Cardiovascular Outcomes. J. Pediatr. 162, S7–S16 (2013).
Cockerill, J., Uthaya, S., Doré, C. J. & Modi, N. Accelerated Postnatal Head Growth Follows Preterm Birth. Arch. Dis. Child Fetal Neonatal. Ed. 91, F184–F187 (2006).
Ehrenkranz, R. A. et al. Longitudinal Growth of Hospitalized Very Low Birth Weight Infants. Pediatrics 104, 280–289 (1999).
Andrews, E. T., Ashton, J. J., Pearson, F., Beattie, R. M. & Johnson, M. J. Early Postnatal Growth Failure in Preterm Infants Is Not Inevitable. Arch. Dis. Child Fetal Neonatal Ed. 104, F235–f241 (2019).
Zeitlin, J. et al. Priorities for Collaborative Research Using Very Preterm Birth Cohorts. Arch. Dis. Child Fetal Neonatal Ed. 105, 538–544 (2020).
Programme, C. A. S. Casp Cohort Study Checklist, <https://casp-uk.b-cdn.net/wp-content/uploads/2018/03/CASP-Cohort-Study-Checklist-2018_fillable_form.pdf> (2018).
Viswanathan, M., Berkman, N. D., Dryden, D. M. & Hartling, L. in Assessing Risk of Bias and Confounding in Observational Studies of Interventions or Exposures: Further Development of the Rti Item Bank (Agency for Healthcare Research and Quality (US), 2013).
Ahn, Y., Sohn, M. & Lee, S. Growth of Korean Preterm Infants in a Family-Centered Tradition During Early Infancy: The Influence of Health Risks, Maternal Employment, and the Sex of Infants. Jpn J. Nurs. Sci. 11, 281–289 (2014).
Bocca-Tjeertes, I. F., Kerstjens, J. M., Reijneveld, S. A., de Winter, A. F. & Bos, A. F. Growth and Predictors of Growth Restraint in Moderately Preterm Children Aged 0 to 4 Years. Pediatrics 128, e1187–e1194 (2011).
Sammy, D. M., Chege, M. N. & Oyieke, J. Early Growth in Preterm Infants after Hospital Discharge in Rural Kenya: Longitudinal Study. Pan Afr. Med. J. 24, 158 (2016).
Teranishi, H., Nakagawa, H. & Marmot, M. Social Class Difference in Catch up Growth in a National British Cohort. Arch. Dis. Child 84, 218–221 (2001).
Holmqvist, P. Growth Increments in Preterm Low Risk Infants. J. Perinat. Med. 19, 285–290 (1991).
Ghods, E., Kreissl, A., Brandstetter, S., Fuiko, R. & Widhalm, K. Head Circumference Catch-up Growth among Preterm Very Low Birth Weight Infants: Effect on Neurodevelopmental Outcome. J. Perinat. Med. 39, 579–586 (2011).
Ni, Y., Beckmann, J., Hurst, J. R., Morris, J. K. & Marlow, N. Size at Birth, Growth Trajectory in Early Life, and Cardiovascular and Metabolic Risks in Early Adulthood: Epicure Study. Arch. Dis. Child Fetal Neonatal Ed. 106, 149–155 (2021).
Kelleher, K. J. et al. Risk Factors and Outcomes for Failure to Thrive in Low Birth Weight Preterm Infants. Pediatrics 91, 941–948 (1993).
Fu, Y. et al. Integration of an Interpretable Machine Learning Algorithm to Identify Early Life Risk Factors of Childhood Obesity among Preterm Infants: A Prospective Birth Cohort. BMC Med. 18, 184 (2020).
Liang, X., Miao, A., Zhang, W., Li, M. & Xing, Y. Effect of Family Integrated Care on Physical Growth and Language Development of Premature Infants: A Retrospective Study. Transl. Pediatr. 11, 965–977 (2022).
Sices, L., Wilson-Costello, D., Minich, N., Friedman, H. & Hack, M. Postdischarge Growth Failure among Extremely Low Birth Weight Infants: Correlates and Consequences. Paediatr. Child Health 12, 22–28 (2007).
Peterson, J., Taylor, H. G., Minich, N., Klein, N. & Hack, M. Subnormal Head Circumference in Very Low Birth Weight Children: Neonatal Correlates and School-Age Consequences. Early Hum. Dev. 82, 325–334 (2006).
Popay, J., et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews: A Product from the Esrc Methods Programme. (2006).
Maukonen, M., Männistö, S. & Tolonen, H. A Comparison of Measured Versus Self-Reported Anthropometrics for Assessing Obesity in Adults: A Literature Review. Scand. J. Public Health 46, 565–579 (2018).
Nuttall, F. Q. Body Mass Index: Obesity, Bmi, and Health: A Critical Review. Nutr. Today 50, 117–128 (2015).
Castillo, E. G. & Harris, C. Directing Research toward Health Equity: A Health Equity Research Impact Assessment. J. Gen. Intern Med. 36, 2803–2808 (2021).
Silva, L. M. et al. Low Socioeconomic Status Is a Risk Factor for Preeclampsia: The Generation R Study. J. Hypertens. 26, 1200–1208 (2008).
Peleg-Oren, N. & Teichman, M. Young Children of Parents with Substance Use Disorders (Sud): A Review of the Literature and Implications for Social Work Practice. J. Soc. Work Pract. Addictions 6, 49–61 (2006).
Smith, T. A., Kievit, R. A. & Astle, D. E. Maternal Mental Health Mediates Links between Socioeconomic Status and Child Development. Curr. Psychol. 42, 21967–21978 (2022).
Swearingen, C., Simpson, P., Cabacungan, E. & Cohen, S. Social Disparities Negatively Impact Neonatal Follow-up Clinic Attendance of Premature Infants Discharged from the Neonatal Intensive Care Unit. J. Perinatol. 40, 790–797 (2020).
MacBean, V., Drysdale, S. B., Zivanovic, S., Peacock, J. L. & Greenough, A. Participant Retention in Follow-up Studies of Prematurely Born Children. BMC Public Health 19, 1233 (2019).
Howe, L. D., Tilling, K., Galobardes, B. & Lawlor, D. A. Loss to Follow-up in Cohort Studies: Bias in Estimates of Socioeconomic Inequalities. Epidemiology 24, 1–9 (2013).
Yi, J. Y., Kim, H. & Chi, I. Urban-Rural Differences in Multimorbidity and Associated Factors in China and Korea: A Population-Based Survey Study. Geriatr. Gerontol. Int 19, 1157–1164 (2019).
DeGuzman, P. B., Huang, G., Lyons, G., Snitzer, J. & Keim-Malpass, J. Rural Disparities in Early Childhood Well Child Visit Attendance. J. Pediatr. Nurs. 58, 76–81 (2021).
Were, V. et al. Comparison of Household Socioeconomic Status Classification Methods and Effects on Risk Estimation: Lessons from a Natural Experimental Study, Kisumu, Western Kenya. Int J. Equity Health 21, 47 (2022).
Hintz, S. R. et al. Neurodevelopmental and Growth Outcomes of Extremely Low Birth Weight Infants after Necrotizing Enterocolitis. Pediatrics 115, 696–703 (2005).
Ng, P. C. The Effectiveness and Side Effects of Dexamethasone in Preterm Infants with Bronchopulmonary Dysplasia. Arch. Dis. Child 68, 330–336 (1993).
Ong, K. K. Size at Birth, Postnatal Growth and Risk of Obesity. Horm. Res. 65, 65–69 (2006).
Cooper, K. & Stewart, K. Does Money Affect Children’s Outcomes? A Systematic Review., (Joseph Rowntree Foundation, 2013).
Padula, A. M. et al. Multilevel Social Factors and NICU Quality of Care in California. J Perinatol 41, 404–412 (2020).
Bohanon, F. J. et al. Race, Income and Insurance Status Affect Neonatal Sepsis Mortality and Healthcare Resource Utilization. Pediatr. Infect. Dis. J. 37, E178–E184 (2018).
Martin, C. R. et al. Nutritional Practices and Growth Velocity in the First Month of Life in Extremely Premature Infants. Pediatrics 124, 649–657 (2009).
Johnson, T. J., Patel, A. L., Bigger, H. R., Engstrom, J. L. & Meier, P. P. Cost Savings of Human Milk as a Strategy to Reduce the Incidence of Necrotizing Enterocolitis in Very Low Birth Weight Infants. Neonatology 107, 271–276 (2015).
Patel, A. L. et al. Impact of Early Human Milk on Sepsis and Health-Care Costs in Very Low Birth Weight Infants. J. Perinatol. 33, 514–519 (2013).
Patra, K. et al. Nicu Human Milk Dose and 20-Month Neurodevelopmental Outcome in Very Low Birth Weight Infants. Neonatology 112, 330–336 (2017).
Smith, M. M., Durkin, M., Hinton, V. J., Bellinger, D. & Kuhn, L. Initiation of Breastfeeding among Mothers of Very Low Birth Weight Infants. Pediatrics 111, 1337–1342 (2003).
Zachariassen, G. et al. Factors Associated with Successful Establishment of Breastfeeding in Very Preterm Infants. Acta Paediatrica 99, 1000–1004 (2010).
Parker, M. G. et al. Approaches to Addressing Social Determinants of Health in the Nicu: A Mixed Methods Study. J. Perinatol. 41, 1983–1991 (2021).
Verbeek, T. et al. Low Socioeconomic Status Increases Effects of Negative Life Events on Antenatal Anxiety and Depression. Women Birth 32, e138–e143 (2019).
Lautarescu, A. et al. Maternal Prenatal Stress Is Associated with Altered Uncinate Fasciculus Microstructure in Premature Neonates. Biol. Psychiatry 87, 559–569 (2020).
Bourque, S. L. et al. The Association of Social Factors and Time Spent in the Nicu for Mothers of Very Preterm Infants. Hosp. Pediatr. 11, 988–996 (2021).
Lindly, O. J. et al. Healthcare Access and Adverse Family Impact among Us Children Ages 0-5 Years by Prematurity Status. Bmc Pediatrics 20, 168 (2020).
Nassel, D. et al. Very Preterm Infants with Technological Dependence at Home: Impact on Resource Use and Family. Neonatology 115, 363–370 (2019).
Argus, B. M., Dawson, J. A., Wong, C., Morley, C. J. & Davis, P. G. Financial Costs for Parents with a Baby in a Neonatal Nursery. J. Paediatr. Child Health 45, 514–517 (2009).
Smith, M. A. & Baum, J. D. Costs of Visiting Babies in Special Care Baby Units. Arch. Dis. Child 58, 56–59 (1983).
Hodek, J. M., von der Schulenburg, J. M. & Mittendorf, T. Measuring Economic Consequences of Preterm Birth - Methodological Recommendations for the Evaluation of Personal Burden on Children and Their Caregivers. Health Econ. Rev. 1, 6 (2011).
Gunn, T. R. et al. Does Early Hospital Discharge with Home Support of Families with Preterm Infants Affect Breastfeeding Success? A Randomized Trial. Acta Paediatr. 89, 1358–1363 (2000).
Holm, K. G. et al. Growth and Breastfeeding of Preterm Infants Receiving Neonatal Tele-Homecare Compared to Hospital-Based Care. J. Neonatal Perinat. Med. 12, 277–284 (2019).
Northrup, T. F., Evans, P. W., Lillie, M. L. & Tyson, J. E. A Free Parking Trial to Increase Visitation and Improve Extremely Low Birth Weight Infant Outcomes. J. Perinatol. 36, 1112–1115 (2016).
Cunningham, C. K., McMillan, J. A. & Gross, S. J. Rehospitalization for Respiratory Illness in Infants of Less Than 32 Weeks’ Gestation. Pediatrics 88, 527–532 (1991).
Slain, K. N., Broberg, M., Stormorken, A., Shein, S. L. & Rotta, A. T. The Cost of Being Poor: The Relationship between Poverty and Hospital Outcomes in Children Critically Ill with Bronchiolitis. Pediatrics, National Conference on Education 2016. United States. 2141 (2011) (no pagination).
Banwell, E. et al. Area Deprivation and Respiratory Morbidities in Children with Bronchopulmonary Dysplasia. Pediatr. Pulmonol. 57, 2053–2059 (2022).
Laugier, O. et al. Influence of Socioeconomic Context on the Rehospitalization Rates of Infants Born Preterm. J. Pediatrics 190, 174–179.e171 (2017).
PEDIATRICS, C. O. C. Poverty and Child Health in the United States. Pediatrics 137 e20160339 (2016).
Cordova-Ramos, E. G. et al. Implementing Social Risk Screening and Referral to Resources in the Nicu. Pediatrics 151, e2022058975 (2023).
Garg, A., Toy, S., Tripodis, Y., Silverstein, M. & Freeman, E. Addressing Social Determinants of Health at Well Child Care Visits: A Cluster Rct. Pediatrics 135, e296–e304 (2015).
Parker, M. G., Garg, A. & McConnell, M. A. Addressing Childhood Poverty in Pediatric Clinical Settings: The Neonatal Intensive Care Unit Is a Missed Opportunity. JAMA Pediatr. 174, 1135–1136 (2020).
Geronimus, A. T. The Weathering Hypothesis and the Health of African-American Women and Infants: Evidence and Speculations. Ethn. Dis. 2, 207–221 (1992).
Sigurdson, K. et al. Racial/Ethnic Disparities in Neonatal Intensive Care: A Systematic Review. Pediatrics 144, e20183114 (2019).
Witt, R. E. et al. Racism and Quality of Neonatal Intensive Care: Voices of Black Mothers. Pediatrics 150, e2022056971 (2022).
Ravi, D., Iacob, A. & Profit, J. Unequal Care: Racial/Ethnic Disparities in Neonatal Intensive Care Delivery. Semin Perinatol. 45, 151411 (2021).
Urban-Rural Europe - Income and Living Conditions. (European Commission, 2022).
Stronks, K., Ravelli, A. C. & Reijneveld, S. A. Immigrants in the Netherlands: Equal Access for Equal Needs? J. Epidemiol. Community Health 55, 701–707 (2001).
Darko, N. Engaging Black and Minority Ethnic Groups in Health Research. (Bristol, UK: Policy Press, 2021).
Lett, E. et al. Health equity Tourism: Ravaging the Justice Landscape. J. Med. Syst. 46, 17 (2022).
Enweronu-Laryea, C. C., Andoh, H. D., Frimpong-Barfi, A. & Asenso-Boadi, F. M. Parental Costs for in-Patient Neonatal Services for Perinatal Asphyxia and Low Birth Weight in Ghana. PLoS One 13, e0204410 (2018).
Organization, W. H. Designing Health Financing Systems to Reduce Catastrophic Health Expenditure. (World Health Organization, 2005).
Koyratty, N. et al. Growth and Growth Trajectory among Infants in Early Life: Contributions of Food Insecurity and Water Insecurity in Rural Zimbabwe. BMJ Nutr. Prev. Health 5, 332–343 (2022).
Khan, K. A. et al. Economic Costs Associated with Moderate and Late Preterm Birth: A Prospective Population-Based Study. Bjog 122, 1495–1505 (2015).
Bango, M. & Ghosh, S. Reducing Infant and Child Mortality: Assessing the Social Inclusiveness of Child Health Care Policies and Programmes in Three States of India. BMC Public Health 23, 1149 (2023).
Jones, L., Mariapun, J., Tan, A. X. Q., Kassim, Z. & Su, T. T. Maternal Wellbeing of Malaysian Mothers after the Birth of a Preterm Infant. BMC Pregnancy Childbirth 23, 510 (2023).
The Evolution of Deficit Thinking: Educational Thought and Practice (The Falmer Press/Taylor & Francis, 1997).
Jenkins, R. H. et al. The Relationship between Austerity and Food Insecurity in the UK: A Systematic Review. EClinicalMedicine 33, 100781 (2021).
Karvonen, K. L. et al. Structural Racism Is Associated with Adverse Postnatal Outcomes among Black Preterm Infants. Pediatr. Res. 94, 371–377 (2023).
Haile, G. A. Precarious Employment and Workplace Health Outcomes in Britain. Soc. Sci. Med. 320, 115694 (2023).
Adkins-Jackson, P. B., Chantarat, T., Bailey, Z. D. & Ponce, N. A. Measuring Structural Racism: A Guide for Epidemiologists and Other Health Researchers. Am. J. Epidemiol. 191, 539–547 (2022).
Kloosterman, G. On Intrauterine Growth. The Significance of Prenatal Care. Int J. Gynaecol. Obstet. 8, 895–912 (1970).
Usher, R. & McLean, F. Intrauterine Growth of Live-Born Caucasian Infants at Sea Level: Standards Obtained from Measurements in 7 Dimensions of Infants Born between 25 and 44 Weeks of Gestation. J. Pediatr. 74, 901–910 (1969).
Fenton, T. R. A New Growth Chart for Preterm Babies: Babson and Benda’s Chart Updated with Recent Data and a New Format. BMC Pediatr. 3, 13 (2003).
de Onis, M. et al. WHO Child Growth Standards. Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight for-Height and Body Mass Index-for-Age, Methods and Development. (World Health Organization, 2006).
Assessment of Differences in Linear Growth among Populations in the Who Multicentre Growth Reference Study. Acta Paediatr. Suppl. 450, 56–65 (2006).
de Onis, M. et al. Development of a Who Growth Reference for School-Aged Children and Adolescents. Bull. World Health Organ 85, 660–667 (2007).
Kramer, M. S. et al. A New and Improved Population-Based Canadian Reference for Birth Weight for Gestational Age. Pediatrics 108, E35 (2001).
Ogden, C. L. et al. Centers for Disease Control and Prevention 2000 Growth Charts for the United States: Improvements to the 1977 National Center for Health Statistics Version. Pediatrics 109, 45–60 (2002).
Roche, A. F., Mukherjee, D., Guo, S. M. & Moore, W. M. Head Circumference Reference Data: Birth to 18 Years. Pediatrics 79, 706–712 (1987).
Ahn, Y., Sohn, M. & Lee, S. [Growth Patterns of Premature Infants up to 40th Term Week of Corrected Age]. J. Korean Acad. Nurs. 41, 613–622 (2011).
Fredriks, A. M. et al. Continuing Positive Secular Growth Change in the Netherlands 1955-1997. Pediatr. Res. 47, 316–323 (2000).
Mattsson, K., Juárez, S. & Malmqvist, E. Influence of Socio-Economic Factors and Region of Birth on the Risk of Preeclampsia in Sweden. Int. J. Environ. Res. Public Health 19, 4080 (2022).
Saumu, W. M. et al. Predictors of Loss to Follow-up among Children Attending Hiv Clinic in a Hospital in Rural Kenya. Pan Afr. Med. J. 32, 216 (2019).
Imbo, A. E., Mbuthia, E. K. & Ngotho, D. N. Determinants of Neonatal Mortality in Kenya: Evidence from the Kenya Demographic and Health Survey 2014. Int J. MCH AIDS 10, 287–295 (2021).
Platt, L. Poverty and Ethnicity in the UK. (2007).
Hjern, A. & Thorngren-Jerneck, K. Perinatal Complications and Socio-Economic Differences in Cerebral Palsy in Sweden - a National Cohort Study. BMC Pediatr. 8, 49 (2008).
Darko, N. (NHS England, 2023).
Wang, Y. et al. Geographical Disparities of Infant Mortality in Rural China. Arch. Dis. Child Fetal Neonatal Ed. 97, F285–F290 (2012).
Funding
K.R. was supported by the University of Southampton National Institute of Health Research Academic Foundation Programme from 2020-2021. A.Y., M.J.J. and R.M.B. are supported by the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre.
Author information
Authors and Affiliations
Contributions
K.R. contributed to the conception, design, data acquisition, data analysis, data interpretation and drafting of the work. A.Y. contributed to the title and abstract screening of articles for inclusion, and to critical revision of the work for important intellectual content. R.M.B. and M.J.J. contributed to the conception and design of the work, and to critical revision of the work for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ravi, K., Young, A., Beattie, R.M. et al. Socioeconomic disparities in the postnatal growth of preterm infants: a systematic review. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03384-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03384-0
- Springer Nature America, Inc.