Abstract
Backgroud
Our study aimed to assess the impact of inter- and intra-observer variations when utilizing an artificial intelligence (AI) system for bone age assessment (BAA) of preschool children.
Methods
A retrospective study was conducted involving a total sample of 53 female individuals and 41 male individuals aged 3–6 years in China. Radiographs were assessed by four mid-level radiology reviewers using the TW3 and RUS–CHN methods. Bone age (BA) was analyzed in two separate situations, with/without the assistance of AI. Following a 4-week wash-out period, radiographs were reevaluated in the same manner. Accuracy metrics, the correlation coefficient (ICC)and Bland-Altman plots were employed.
Results
The accuracy of BAA by the reviewers was significantly improved with AI. The results of RMSE and MAE decreased in both methods (p < 0.001). When comparing inter-observer agreement in both methods and intra-observer reproducibility in two interpretations, the ICC results were improved with AI. The ICC values increased in both two interpretations for both methods and exceeded 0.99 with AI.
Conclusion
In the assessment of BA for preschool children, AI was found to be capable of reducing inter-observer variability and enhancing intra-observer reproducibility, which can be considered an important tool for clinical work by radiologists.
Impact
-
The RUS-CHN method is a special bone age method devised to be suitable for Chinese children.
-
The preschool stage is a critical phase for children, marked by a high degree of variability that renders BA prediction challenging.
-
The accuracy of BAA by the reviewers can be significantly improved with the aid of an AI model system.
-
This study is the first to assess the impact of inter- and intra-observer variations when utilizing an AI model system for BAA of preschool children using both the TW3 and RUS-CHN methods.
Similar content being viewed by others
Introduction
Bone age (BA), an indicator of biological age,1,2,3 is determined through the assessment of hand-wrist X-rays to gauge skeletal maturity. This assessment serves as a reflection of the actual growth and development in children. The disparity between skeletal age and chronological age (CA) is pivotal in bone age assessment (BAA) for monitoring growth irregularities in children, verifying endocrine-related diagnoses, forecasting adult height, and appraising treatment efficacy.1 In China, two noteworthy BAA methods are employed, specifically the Tanner–Whitehouse III (TW3) method.4 and the China 05 RUS–CHN (RUS–CHN) method.5 Presently, the TW3 method stands as the globally prevalent BAA method.6 This scoring system having undergone two modifications, evaluates the skeletal maturity of individuals.4 The RUS-CHN method, introduced by Chinese researchers in 2005, was devised to be more suitable for Chinese children compared to the globally adopted TW3 method.5 Consequently, the development of a high-performance automatic assessment system that combines these two BA evaluation methods holds substantial clinical significance.
Computer hardware capabilities advance and software technology continually improves, the integration of artificial intelligence (AI) technology in the medical domain has grown increasingly commonplace.7 Over recent years, deep learning (DL) models founded on extensive data have played pivotal roles in various aspects of disease diagnosis.8,9 Given the relatively straightforward nature of BAA, which entails the assessment of a single wrist X-ray image utilizing a standardized scoring system, it represents an ideal avenue for training DL solutions and crafting AI model systems. Multiple AI systems related to BA have been developed globally, including BoneXpert, GoogLeNet, and OxfordNet. These systems yield results comparable to traditional manual BAA, offering the merits of objectivity, efficiency, and time-saving.8,9,10,11 Numerous studies have demonstrated that AI application in BAA enhances the diagnostic accuracy of radiologists while reducing evaluation time.10,12,13 Some of these studies have concentrated on the assessment consistency among radiologist observers although only a limited number have delved into intra-observer variability. The preschool stage is a critical phase for monitoring the growth and development of children in China,14 marked by a high degree of variability that renders BA prediction challenging. Insufficient studies employed the TW3 and RUS-CHN methods for assessing the BA of preschool children.
Hence, the selection of an AI model system capable of assisting radiologists in BAA is crucial. The DL software we employ can accommodate a range of BAA methods, encompassing both the TW3 and RUS-CHN methods, for appraising the hand-wrist of BA in preschool children. This study’s objective is to investigate the impact of AI model software on inter-observer consistency and intra-observer reproducibility in X-ray BAA for preschool children.
Materials and methods
Data acquisition
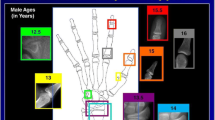
This retrospective study involved the selection of 471 left hand-wrist radiographs sourced from the Third Affiliated Hospital of Zhejiang Chinese Medicine University in Hangzhou, China. The radiographs were all retrieved from the Picture Archive and Communication Systems (PACS) spanning the period between January 2018 and December 2022. The inclusion criteria stipulated: 1) children aged between 3 and 6 years, 2) children of Chinese nationality and residing in China, and 3) children with no significant medical history of conditions affecting skeletal development. Unclear left hand-wrist radiographs were excluded from the study. We performed stratified random sampling among preschool children aged 3–6, with 20% of the data from each age group included in the study sample. This resulted in a total of 53 female individuals and 41 male individuals (Fig.1, Table 1). None of the cases in the study were involved in the training and validation of the AI model system.
Imaging examination method
All medical images were captured using the Canon CMP200 scanner from Canon Medical Systems scanner. Radiographs of the left hand-wrist were acquired using the following exposure settings: 1) Tube voltage at 75 kV, tube current at 200 uA, film distance approximately 90 cm, and exposure time of 500 ms. 2) The subject’s left palm was positioned at the center of the irradiation field, pressed firmly against the detection plate, fingers naturally extended, and the centerline aligned with the base of the third metacarpal bone. 3) The displayed area encompassed all carpal, metacarpal, and phalangeal bones, in addition to the distal radius and ulna, covering a range of 3–4 cm. 4) Radiation protection measures were taken to safeguard other body parts.
Bone age assessment
Radiograph evaluation
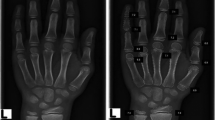
The BA films were stored in Digital Imaging and Communications in Medicine format within the PACS, with the subject’s information anonymized. All readers were kept unaware of the clinical medical history and patient characteristics. Each radiograph for BAA was assessed using two methods: 1) the TW3 method, which scores the skeletal maturity of each hand and wrist bone, as published in 2001,4 and 2) the RUS–CHN method, which analyzes the skeletal development standards of the hand and wrist for Chinese children, version 05-I, as published in 2006.5
Reference bone age
Two associate chief radiology physicians, trained in the BAA system, were recruited for this study. Each of them possessed over 15 years of experience in BAA and evaluated more than 2000 films annually. They conducted BAA for the hand-wrist radiographs in the samples, following a double-blind approach, and obtained BA values using both the TW3 method and RUS-CHN method. The average of their results was calculated to establish the reference BA standard for this study.
AI model development for BAA
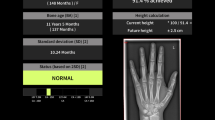
The AI model system named Dr. Wise for BAA in this study was developed by the DeepWise Inc. The software had received clinical approval from China’s National Medical Products Administration for Class III medical device certification in 2022 and gained widespread acceptance for clinical use. Hand and wrist landmark detection was automatically performed by this software to identify region of interests for epiphyseal development ratings. Users could overwrite the initial BAA proposed by AI models. Previous study disclosed that the AI model was trained using more than ten thousand hand radiographs from six centers in China.15 The mean absolute error (MAE) between the Dr. Wise AI model’s results and the reference standard was 0.266 and 0.249 years for the TW3 method and the RUS-CHN method, respectively.
Radiograph interpretation
A cross-study design was adopted for radiograph interpretation. Four mid-level radiologists, certified by the committee and possessing 3–5 years of experience, were selected as reviewers. They underwent training on BAA systems before conducting assessments on the samples, familiarizing themselves with the reading and reporting protocols. Following a double-blind approach to image evaluation, they performed BAA using both the TW3 method and the RUS–CHN method.
All researchers carried out two radiograph interpretations, with a 4-week wash-out period separating the two interpretations. To minimize memory-related errors, a two-step random cross-reading method was employed for each interpretation. The sample database’s radiographs were randomly divided into two portions: one for interpretation with AI assistance and the other for interpretation without AI. Radiographs were independently evaluated by reviewers under different conditions. Reviewers were informed of the sex and chronological age of subjects, but were blinded of each other’s BAA results, as in the case of their daily clinical practices. Bone age assessment was conducted by reviewers relying on their own experience, and results were directly derived and recorded. While in the interpretation with AI, the reviewers assessed bone age through the following 3 steps: 1) Reviewers were asked to undergo an independent process of BAA. 2) The BAA results generated by the AI model system were then sent to the reviewers’ computers. 3) Reviewers were guided to make comparations and corrections of the previous independent results, and complete the final BAA report with the assistance of AI. A 2-week wash-out period was maintained between the two steps. The crossover study design is depicted in Figs. 1 and 2.
Data analysis
Data analysis was conducted using SPSS statistical software (version 26.0, SPSS, Inc, Chicago, IL). With the reference standard’s average value as the basis for comparison, the root mean square error (RMSE), MAE, and the percentage of errors within 0.50 years and 0.25 years for the first BAA interpretation were calculated and compared among the four mid-level radiologists under the conditions of “no AI” and “with AI,” respectively. Quantitative data were examined for normality using histograms. Paired t-tests were used to compare the MAEs of reviewers with and without AI, with significance set at a p-value < 0.001. ICCs, Bland-Altman plots with the mean difference and 95% limits of agreement (LoA) results were generated to compare the results with and without AI for the four reviewers (Reviewers 1–4) in the 1st and 2nd interpretations. Intra-observer reproducibility among reviewers was assessed using the ICCs and 95% LoA results, comparing BAA results of the same reviewer in two different interpretations.
Results
Accuracy of AI model system in BAA
The results of BAA using the TW3 method and RUS-CHN methods, both with and without AI assistance, were compared with the reference standard, as shown in Fig. 3. In the first interpretation, the accuracy of the TW3 method’s results for BAA improved significantly with AI assistance compared to without AI. RMSE decreased from 0.358 to 0.151 and MAE reduced from 0.325 to 0.119 (p < 0.001). The accuracy within 0.50 years increased from 83.5% to 99.7%, and the accuracy within 0.25 years rose significantly from 21.5% to 84.3%. Similarly, when utilizing the RUS-CHN method for BAA, the results’ accuracy was enhanced with AI assistance compared to without AI. RMSE decreased from 0.359 to 0.148 and MAE decreased from 0.309 to 0.113 (p < 0.001). The accuracy within 0.50 years increased from 83.8% to 99.2%, and the accuracy within 0.25 years increased significantly from 31.4% to 85.9%.
Comparison of inter-observer agreement
In the inter-observer agreement comparison for the TW3 method, the ICC values, mean differences and 95% LoA results of the four reviewers improved when using the AI model system. Their ICC values increased from 0.956 to 0.991 in the first interpretation and from 0.974 to 0.993 in the second interpretation. Their mean difference and 95% LoA results increased from −0.17 (95% LoA: −0.79, 0.45) to −0.01 (95% LoA: −0.26, 0.24) in the first interpretation and from −0.15 (95% LoA: −0.62, 0.32) to −0.05 (95% LoA: −0.33, 0.22) in the second interpretation. In the case of the RUS-CHN method, the ICC values, the mean difference and 95% LoA results also saw improvements. Their ICC values increased from 0.950 to 0.991 in the first interpretation and from 0.963 to 0.996 in the second interpretation with AI assistance. Their mean difference and 95% LoA results increased from −0.15 (95% LoA: −0.79, 0.48) to −0.05 (95% LoA: −0.32, 0.22) in the first interpretation and from −0.11 (95% LoA: −0.66, 0.43) to −0.03 (95% LoA: −0.21, 0.15) in the second interpretation with AI assistance (Fig. 4).
Comparison of intra-observer reproducibility
In the two interpretations, the intra-observer reproducibility of the TW3 method showed that the ICC and 95% LoA results for each reviewer with the assistance of the AI model system were slightly improved than without AI. Similar results were observed when using the RUS-CHN method for BAA. Furthermore, with AI assistance for BAA, the ICCs for both methods among all reviewers exceeded 0.99, and the mean differences were close to zero (Figs. 5 and 6).
Discussion
Bone age serves as a quantitative measure of skeletal development maturity.1 The utilization of X-ray wrist images for BAA in children become widespread.10,11,12,13,14 In the past, manual BAA methods required observers to meticulously compare or score individual bones.16 DL offers a faster and more consistent solution. We categorized multiple observers into groups, scrutinized and compared their diagnostic accuracy both with and without AI assistance, and assessed inter-observer consistency and intra-observer reproducibility. The findings reveal that experienced radiologists can enhance the precision of BAA with the aid of AI. Simultaneously, AI can mitigate inter-observer variability and enhance intra-observer reproducibility.
AI technology stands as a prominent application within the realm of medical imaging, including the diagnosis of lung nodules and the detection of bladder cancer.17,18 DL can precisely quantify the shape and position of each target bone in the wrist for BAA, with its development dating back to 2017.19 Presently, researchers construct algorithmic models to predict BA rapidly and accurately.20 drawing from a vast repository of images.9,21 Spampinato et al.9 were pioneers in exploring the application of DL to medical images, and they demonstrated an average deviation of about 0.8 years when compared to manual evaluations. In 2020, Reddy et al.22 employed a publicly provided anonymized dataset from the Radiological Society of North America pediatric bone age challenge.2 The MAEs between the models for the whole hand and index finger were comparable (0.392 years vs. 0.425 years, p = 0.14). Both BA values were significantly smaller than those obtained by three pediatric radiologists from single-finger radiographs (0.667 years, p < 0.0001). Larson et al.21 developed a DL model for BAA based on a comparison with 12,611 clinical hand radiographs using the Greulich and Pyle (GP) atlas and corresponding clinical radiology reports. The mean difference between the model’s BAA radiographs and reviewers was 0 years, with a mean RMSE and MAE of 0.63 and 0.50 years, respectively. All assessments fell within the 95% limits of agreement with each other. The Residual Network model effectively extracts X-ray bone image features and autonomously determines bone age, boasting an impressive BA prediction accuracy of 97.6% and a MAE of 0.455 year.12 AI models have consistently demonstrated high accuracy in BAA,21,22,23 and this study’s results reaffirm this fact. Radiologists can enhance their diagnostic accuracy in BA evaluation with the assistance of AI models.
Environmental and ethnic factors exert varying degrees of influence on bone development, leading to differing outcomes in BAA. We employed two distinct BAA methods, primarily suited for Chinese children. Both the TW3 method and the RUS-CHN method are widely utilized for the assessment of preschool children. The TW3 method evaluates and scores the maturity of each region of interest bone and drew reference data from children residing in Europe and America, with publication occurring in 2001.4 The TW3 method is a quantitative approach that scores and sums 20 hand-wrist bones, which characterized by strong objectivity, resulting in highly accurate assessments with a precision of less than one month.24 However, it is time-consuming and entails a complex evaluation process. Several studies have affirmed the high accuracy of the TW3 method for BAA has high accuracy.3,25 In a British children’s sample, CA was underestimated in females beyond the age of 3 years, resulting in significant differences between BA and CA (−0.43 years, p < 0.001), while no such differences were observed in males (0.01 years, p = 0.760).3 Based on an analysis of 9059 clinical left hand radiographs, an optimized TW3-AI system for BAA exhibited strong concordance with the overall assessment of reviewers, with a RMSE of 0.50 years.25 In our study, with the aid of the AI model system, the RMSE of observations by mid-level doctors decreased from 0.358 to 0.151. This further underscores that AI has the potential to narrow the disparity in BAA results compared to the reference standard in the TW3 method, thereby assisting physicians in enhancing diagnostic accuracy. In 2006, researchers5 revised the standards based on the TW3 method and established the RUS-CHN method. using samples from urban areas in China Building on the original bone development framework of the TW3 method, the RUS-CHN method identifies new maturity characteristics, which better align with the actual skeletal conditions of children during their rapid growth and development. It also subdivides the long-term fusion process of the radius and ulna into five distinct grades, thereby enhancing accuracy throughout the entire growth and development period.26 The RUS-CHN method, necessitates more steps, consumes additional time during the evaluation process, and is challenging to master. In a preliminary study conducted by our team involving 390 preschool children, it was observed that while the TW3 method outperformed the RUS-CHN method, it was not entirely reliable on its own. This is because both methods tended to overestimate the age of both sexes. Nevertheless, the median difference of the TW3 method approached zero.27 In the current study, when observers used the RUS-CHN method, both with and without AI assistance, the RMSE was 0.359 and 0.148, while the MAE was 0.309 and 0.113, respectively, signifying a high level of diagnostic performance. Moreover, with the aid of AI, observer diagnostic accuracy can be further enhanced.
Applying AI systems to BAA presents two primary challenges, namely ensuring consistency in both inter- and intra-observer evaluations. in an investigation involving American children, researchers compared the BAA performance of a group of pediatric radiologists with and without AI support. With AI assistance, BAA accuracy improved, with an overall accuracy of 68.2% compared to 63.6%, and an accuracy of 98.6% within 1 year compared to 97.4%. Additionally, the ICC with AI was 0.9951, whereas without AI, it was 0.9914.10 Lee KC et al.28 discovered that a deep learning-based model exhibited accuracy in BAA for a total of 102 hand radiographs. Furthermore, it appeared to enhance clinical efficacy by improving inter-observer reliability, which slightly increased the ICC of the two observers from 0.945 to 0.990 with AI. More recently, Wang X et al.15 concluded that an AI model enhances both the accuracy and consistency of BAA for physicians of all experience levels. The accuracies of senior, mid-level, and junior physicians were significantly better with AI assistance than without AI assistance (MAEs of 0.325, 0.344, and 0.370 vs. 0.403, 0.469, and 0.755, respectively). Moreover, their consistency results were significantly higher with AI assistance than without AI assistance (ICCs of 0.996, 0.996, and 0.992 vs. 0.987, 0.989, and 0.941, respectively). In this study, for the inter-observer agreement comparison, with the aid of AI, the ICC values for both BAA methods reached 0.991 in the 1st interpretation. Regarding intra-observer reproducibility between the 1st and 2nd interpretation, the ICC results were elevated to 0.998 for the TW3 method and up to 0.997 for the RUS-CHN method (Reviewer 4). And the Bland-Altman plots showed an excellent agreement among the reviewers in both two methods. The Utilizing AI-assisted software in BAA can help reviewers mitigate both inter-observer variability and intra-observer variability.
The development of AI software has simplified and expedited the BAA process. Numerous studies have compared BAA differences between AI tools and radiologists.13,16,21,28,29,30 Their findings confirm that AI can enhance diagnostic accuracy. However, relying solely on AI results without confirmation from a radiologist is not considered reliable.31 In such cases, AI software is designed to assist radiologists in making faster and more accurate diagnoses rather than replacing radiologists outright. two scenarios were established for observers, one with and one without the AI model system, and BAA accuracy was calculated separately. Our results align with previous findings and further substantiate that AI can help radiologists enhance the accuracy of BAA, particularly in preschool children, using both the TW3 and RUS-CHN methods.
The present study has several limitations: 1) This is a single-center, cross-sectional study with a small sample size, focused only on a specific population aged 3–6 years in China. 2) The study exclusively compared the TW3 and RUS-CHN methods, but other methods like the GP method, which is commonly used in various regions and hospitals, were not considered. 3) The observers in this study were mid-level attending physicians, and there was no comparison with physicians of other levels, such as junior and senior physicians. 4) The timing of bone age assessment was not documented, even though previous studies have found that AI can reduce assessment time. Comparative time consumption should be considered. Therefore, more in-depth multicenter studies are necessary to validate these findings, incorporating various BAA methods and observers with different levels of experience in future research.
During the process of BAA for preschool children, the use of AI model systems can significantly improve not only the diagnostic accuracy of physicians but also the consistency among observers and the reproducibility within observers. As a result, AI model systems hold great promise for X-ray hand-wrist bone age assessment and are a valuable tool in the clinical work of radiologists.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
References
Creo, A. L. & Schwenk, W. F. 2nd Bone Age: A Handy Tool for Pediatric Providers. Pediatrics 140, e20171486 (2017).
Halabi, S. S. et al. The Rsna Pediatric Bone Age Machine Learning Challenge. Radiology 290, 498–503 (2019).
Alshamrani, K. & Offiah, A. C. Applicability of Two Commonly Used Bone Age Assessment Methods to Twenty-First Century Uk Children. Eur. Radio. 30, 504–513 (2020).
Tanner, J. M., Healy, M. J., Goldstein, H. & Cameron, N. Assessment of skeletal maturity and prediction of adult height: TW3 method, 3rd ed. (W.B. Saunders, 2001).
Zhang, S. Y. et al. Reference Values of Differences between Tw3-C Rus and Tw3-C Carpal Bone Ages of Children from Five Cities of China. Zhonghua er ke za zhi Chin. J. Pediatrics 46, 851–855 (2008).
Kowo-Nyakoko, F. et al. Evaluation of Two Methods of Bone Age Assessment in Peripubertal Children in Zimbabwe. Bone 170, 116725 (2023).
Nicholas, J. L. et al. Us Evaluation of Bone Age in Rural Ecuadorian Children: Association with Anthropometry and Nutrition. Radiology 296, 161–169 (2020).
Zhang, Y. et al. Smanet: Multi-Region Ensemble of Convolutional Neural Network Model for Skeletal Maturity Assessment. Quant. Imaging Med. Surg. 12, 3556–3568 (2022).
Chen, C. et al. Attention-Guided Discriminative Region Localization and Label Distribution Learning for Bone Age Assessment. IEEE J. Biomed. Health Inf. 26, 1208–1218 (2022).
Tajmir, S. H. et al. Artificial Intelligence-Assisted Interpretation of Bone Age Radiographs Improves Accuracy and Decreases Variability. Skelet. Radio. 48, 275–283 (2019).
Zhao, K. et al. Effect of Ai-Assisted Software on Inter- and Intra-Observer Variability for the X-Ray Bone Age Assessment of Preschool Children. BMC Pediatr. 22, 644 (2022).
Han, Y. & Wang, G. Skeletal Bone Age Prediction Based on a Deep Residual Network with Spatial Transformer. Comput. Methods Prog. Biomed. 197, 105754 (2020).
Oza, C. et al. A Comparison of Bone Age Assessments Using Automated and Manual Methods in Children of Indian Ethnicity. Pediatr. Radio. 52, 2188–2196 (2022).
Zhao, X. et al. Construction of Artificial Intelligence System of Carpal Bone Age for Chinese Children Based on China-05 Standard. Med. Phys. 49, 3223–3232 (2022).
Wang, X. et al. Artificial Intelligence-Assisted Bone Age Assessment to Improve the Accuracy and Consistency of Physicians with Different Levels of Experience. Front. Pediatr. 10, 818061 (2022).
Wang, F. et al. Artificial Intelligence System Can Achieve Comparable Results to Experts for Bone Age Assessment of Chinese Children with Abnormal Growth and Development. PeerJ 8, e8854 (2020).
Lee, J. H., Hong, H., Nam, G., Hwang, E. J. & Park, C. M. Effect of Human-Ai Interaction on Detection of Malignant Lung Nodules on Chest Radiographs. Radiology 307, e222976 (2023).
Wu, S. et al. Artificial Intelligence-Based Model for Lymph Node Metastases Detection on Whole Slide Images in Bladder Cancer: A Retrospective, Multicentre, Diagnostic Study. Lancet Oncol. 24, 360–370 (2023).
Spampinato, C., Palazzo, S., Giordano, D., Aldinucci, M. & Leonardi, R. Deep Learning for Automated Skeletal Bone Age Assessment in X-Ray Images. Med. Image Anal. 36, 41–51 (2017).
Foersch, S. et al. Multistain Deep Learning for Prediction of Prognosis and Therapy Response in Colorectal Cancer. Nat. Med. 29, 430–439 (2023).
Larson, D. B. et al. Performance of a Deep-Learning Neural Network Model in Assessing Skeletal Maturity on Pediatric Hand Radiographs. Radiology 287, 313–322 (2018).
Reddy, N. E. et al. Bone Age Determination Using Only the Index Finger: A Novel Approach Using a Convolutional Neural Network Compared with Human Radiologists. Pediatr. Radio. 50, 516–523 (2020).
Booz, C. et al. Artificial Intelligence in Bone Age Assessment: Accuracy and Efficiency of a Novel Fully Automated Algorithm Compared to the Greulich-Pyle Method. Eur. Radio. Exp. 4, 6 (2020).
Tanner. J. M. Growth at adolescent, 2nd ed. (Blackwell Scientific Publications, 1962). Available at http://www.blackwellpublishing.com/.
Zhou, X. L. et al. Diagnostic Performance of Convolutional Neural Network-Based Tanner-Whitehouse 3 Bone Age Assessment System. Imaging Med Surg. 10, 657–667 (2020).
Zhang, S. Y. et al. Standards of Tw3 Skeletal Maturity for Chinese Children. Ann. Hum. Biol. 35, 349–354 (2008).
Gao, C. et al. A Comparative Study of Three Bone Age Assessment Methods on Chinese Preschool-Aged Children. Front. Pediatr. 10, 976565 (2022).
Lee, K. C. et al. Clinical Validation of a Deep Learning-Based Hybrid (Greulich-Pyle and Modified Tanner-Whitehouse) Method for Bone Age Assessment. Korean J. Radio. 22, 2017–2025 (2021).
Bowden, J. J. et al. Validation of Automated Bone Age Analysis from Hand Radiographs in a North American Pediatric Population. Pediatr. Radio. 52, 1347–1355 (2022).
Chávez-Vázquez, A. G. et al. Evaluation of Height Prediction Models: From Traditional Methods to Artificial Intelligence. Pediatr. Res. 95, 308–315 (2024).
Offiah, A. C. Current and Emerging Artificial Intelligence Applications for Pediatric Musculoskeletal Radiology. Pediatr. Radio. 52, 2149–2158 (2022).
Acknowledgements
We express our gratitude to all the children and their parents or guardians for their cooperation and willingness to participate in this study.
Funding
This study was funded by the Medical and Health Science and Technology Fund of Zhejiang Province (No. 2020PY014), the Chinese Medicine Science and Technology Plan for the Modernization of Traditional Chinese Medicine Fund of Zhejiang Province (No. 2021ZX011) and the Chinese Medicine Science and Technology Fund of Zhejiang Province (No. 2019ZQ031).
Author information
Authors and Affiliations
Contributions
CG, QQ, ML and ZD conceived and designed the study, had full access to the data in the study and took responsibility for the integrity of the data and the accuracy of data analysis. CG, CH, ML and ZD drafted the paper. CG, CH, YL, XX and PG analyzed the data. CG, XX and PG contributed to data acquisition. All authors critically revised the manuscript for intellectual content and approved the version for publication. All authors agree to be accountable for all aspects of the work related to the accuracy or integrity for appropriate investigation and resolution of any queries.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study involving human participants was reviewed and approved by the Medical Research Ethics Committee of the Third Affiliated Hospital of Zhejiang Chinese Medicine University, China. Written informed consent was provided by the participants’ legal guardians or next of kin.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, C., Hu, C., Qian, Q. et al. Artificial intelligence model system for bone age assessment of preschool children. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03282-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03282-5
- Springer Nature America, Inc.