Abstract
Background/Objective
To identify predictors of incident type 2 diabetes using a mixed meal tolerance test (MMTT).
Methods
Adult Indigenous Americans without diabetes (n = 501) from a longitudinal cohort underwent at baseline a 4-h MMTT, measures of body composition, an oral glucose tolerance test, an intravenous glucose tolerance test for acute insulin response (AIR), and a hyperinsulinemic-euglycemic clamp for insulin action (M). Plasma glucose responses from the MMTT were quantified by the total and incremental area under the curve (AUC/iAUC).
Results
At follow-up (median time 9.6 [inter-quartile range: 5.6–13.5] years), 169 participants were diagnosed with diabetes. Unadjusted Cox proportional hazards models, glucose AUC180-min (HR: 1.98, 95% CI: 1.67, 2.34, p < 0.0001), AUC240-min (HR: 1.93, 95% CI: 1.62, 2.31, p < 0.0001), and iAUC180-min (HR: 1.43, 95% CI: 1.20, 1.71, p < 0.0001) were associated with an increased risk of diabetes. After adjustment for covariates (age, sex, body fat percentage, M, AIR, Indigenous American heritage) in three subsequent models, AUC180-min (HR: 1.44, 95% CI: 1.10, 1.88, p = 0.007) and AUC240-min (HR: 1.41, 95% CI: 1.09, 1.84, p < 0.01) remained associated with increased risk of diabetes.
Conclusions
Glucose responses to a mixed meal predicted the development of type 2 diabetes. This indicates that a mixed nutritional challenge provides important information on disease risk.
Clinical Trial Registry
ClinicalTrials.gov identifier : NCT00340132, NCT00339482
Similar content being viewed by others
Introduction
Type 2 diabetes is one of the most prevalent global chronic diseases, and in the United States an estimated 37.3 million people have diabetes, with 90–95% of cases being type 2 [1]. In the last several decades, research has evaluated the impact of nutrition and dietary intake on type 2 diabetes, both as a risk factor and a means of glycemic control [2]. However, the wide variation in individual metabolic responses to diet has prompted the development of individualized weight-loss plans and has subsequently ushered in the era of precision nutrition, which aims to use multi-dimensional data from analytical computing platforms to provide targeted recommendations to an individual based upon a clustering of factors including response to diet and other variables such as genetics, gut microbiome, clinical assays (e.g., lipid profile), and lifestyle measures (e.g., sleep, physical activity) [3,4,5]. Although the oral glucose tolerance test (OGTT) has been historically the clinical standard for diagnosing and categorizing changes in glucose metabolism, glycemic phenotypes (e.g. impaired fasting glucose versus impaired glucose tolerance), and diabetes, a mixed meal tolerance test (MMTT) is more akin to daily intake as foods are often solid and there is a macronutrient mix of carbohydrates, fats and protein which each uniquely stimulate insulin secretion [4, 6,7,8,9]. Compared with an OGTT, an MMTT represents a substantively different nutritional challenge.
Previous research has noted the importance of measuring postprandial glucose and insulin responses to substrates such as lipids to determine individual variability and how that may impact the risk of developing diabetes and disease [10]. Since an MMTT perturbs more systems [3] and may provide more physiologically relevant metabolic responses than an OGTT [9], the utility of an MMTT as a precision nutrition tool is under active investigation. For example, machine-learning models incorporating high-dimensional data have been developed to predict glucose and insulin responses after a mixed meal [5]. In addition, the U.S. National Institutes of Health has recently funded a large multicenter study of approximately 10,000 participants seeking to implement Artificial Intelligence-driven algorithms and dietary response including a single mixed-meal test [11]. The usefulness of these studies is enhanced if postprandial glucose and insulin concentrations provide information about future health outcomes. However, MMTT is not as well established at informing disease risk including the risk of diabetes. Therefore, the aim of this analysis was to assess if glucose and insulin responses to an MMTT can predict the development of type 2 diabetes.
Methods
Study design
Individuals who participated in a longitudinal study of the pathogenesis of type 2 diabetes (NCT00339482) [12] and participated in a detailed, inpatient metabolic study assessing determinants of type 2 diabetes (NCT00340132) [13,14,15] were included in the current analyses. Both studies were approved by the Institutional Review Board of the National Institute of Diabetes and Digestive Kidney Diseases. Prior to participation, volunteers were informed of the nature, purpose, and risks of both studies and written informed consent was obtained from all participants for both studies. Adult participants who were not taking medications known to affect glucose metabolism, did not have type 2 diabetes at baseline, and determined to be healthy based on medical history, physical examination, and routine screening laboratory tests were admitted to our clinical research unit in Phoenix, Arizona from 1982 to 2007. During the inpatient baseline visit participants were administered a weight-maintaining diet (50% carbohydrate, 30% fat, 20% protein) as previously described [16]. The inpatient visit included measurements (described below) of body composition, MMTT, IVGTT, hyperinsulinemic-euglycemic clamp (HIEC), and an OGTT to verify the absence of type 2 diabetes during the baseline inpatient visit. Following the inpatient visit, participants who also participated in the longitudinal study were seen approximately every 2 years for outpatient visits during which an OGTT was performed to assess diabetes status. Diabetes and diagnosis date were determined by OGTT values at the time of the outpatient visit or from a review of medical records [14, 15]. Classification of diabetes was based on the 2003 American Diabetes Association criteria [17].
Body composition
Body composition was assessed by underwater weighing with determination of residual lung volume by helium dilution or dual-energy X-ray absorptiometry (DXA; DPX-L and Lunar Prodigy, GE Lunar, Madison, WI). Body composition measures were made comparable using previously derived equations [18, 19].
MMTT
After an overnight fast at 0730 h, participants consumed a standardized breakfast containing 30% of their respective weight-maintaining energy requirements [20, 21]. A subset of participants consumed an additional standardized lunch meal at 1130 h. Meals were composed of 20% protein, 40% carbohydrate, and 40% fat. Blood samples for plasma glucose and insulin were drawn at −15 and 0 min prior to the start of the breakfast meal and thereafter every 30 min for up to 240 minutes. The total and incremental areas under the curve (AUC and iAUC, respectively) for plasma glucose and insulin were calculated with the linear trapezoidal rule [22]. The iAUCs were calculated by subtracting the baseline area from the total AUC. AUC/iAUCs were calculated in two ways (1) 0–180 min and (2) 0–240 min. The peak glucose and insulin values between fasting and 240 min, rise from fasting to peak (Δ = peak – fasting value), and decline from peak to 240 min (Δ = 240 minutes value – peak) were also calculated for each individual.
OGTT and IVGTT
Participants underwent a 75-g OGTT with venous glucose measurements during the baseline inpatient visit and subsequent outpatient follow-up visits. Acute insulin response (AIR), a measure of insulin secretion, was calculated using 25-g intravenous glucose bolus injected over 3 min and calculated as the mean incremental plasma insulin concentration from the 3rd to 5th minute after injection [15, 21]. The AUC/iAUCs for glucose and insulin during the OGTT were also calculated.
Hyperinsulinemic-euglycemic clamp (HIEC)
Insulin action was measured using a hyperinsulinemic-euglycemic clamp (HIEC), as previously described [23]. In brief, after an overnight fast, a primed continuous insulin infusion (240 pmol/m2/min based on body surface area) was administered for 100 min with a 20% dextrose solution infused at varying rates to maintain a 5.55 mmol/L plasma glucose concentration. The rate of total insulin-stimulated glucose disposal (M) was calculated for the last 40 min of the insulin infusions and corrected for steady-state insulin plasma concentrations and endogenous glucose output. Endogenous glucose output was determined via a primed continuous [3-3H] glucose infusion (0.3 µCi/min) prior to (for 120 minutes) and during the insulin infusion. HIEC measures were normalized to estimated metabolic body size (fat-free mass + 17.7 kg) [24].
Statistical Analyses
Analyses were performed in SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Normally distributed data are presented as mean ± standard error of the mean (SEM), while skewed distributions are reported as median with interquartile range (IQR). Insulin AUC/iAUCs, AIR and M were log transformed to approximate a normal distribution. Surrogate measures of insulin secretion were calculated as ∆ insulin 0 to 30 min/∆ glucose 0 to 30 min and insulin resistance as 1/fasting insulin and multiplied to get the disposition index (DI) [25]. Descriptive statistics of baseline characteristics were compared between participants who were and were not diagnosed with diabetes by the end of the study. Differences between groups (diabetes vs. no diabetes) were assessed using independent sample t-tests for continuous variables and chi-square tests for categorical variables. Paired t-tests were used to compare glucose and insulin AUCs.
Cox proportional hazards models were used to assess the prospective relationship between MMTT glucose and insulin responses and development of diabetes. A series of progressively adjusted models were fit [1]: unadjusted [2]; adjusted for age, sex, body fat (%) [3]; further adjusted for M [4]; further adjusted for AIR and full vs. non-full Southwestern Indigenous American heritage (SWIA). For all insulin measures a final set of models including the corresponding glucose measure were assessed; however, results remained unchanged (results not shown). Proportional hazards assumptions were assessed using Schoenfeld residuals. To facilitate comparisons, all continuous variables were standardized (i.e. mean = 0, SD = 1) and the hazard ratios (HR) were reported per SD.
Pearson correlation coefficients were utilized to assess the bivariate associations between MMTT and OGTT variables. Prediction models of diabetes, accounting for time to event, enabled calculation of C-statistics to compare the predictive abilities of MMTT variables with the corresponding OGTT variable, fasting glucose, 60-min glucose and 120-min glucose from the OGTT [14, 26]. Fasting and 120-min glucose from the OGTT were included as they are known predictors of diabetes. C-statistics were quantified using the Pencina and D’Agostino method and compared using the DeLong method [27, 28]. C-statistics were calculated for significant model 4 measures.
Results
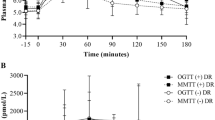
Of the 501 participants, 169 (34%) were diagnosed with type 2 diabetes with median follow-up time of 9.6 years (IQR: 5.6–13.5 years). Demographic characteristics are reported in Table 1. Adults who developed type 2 diabetes were more likely to be female, had higher body mass indices (37.0 ± 0.5 kg/m2 versus 31.8 ± 0.4 kg/m2) and body fat (36.2 ± 0.6% versus 31.2 ± 0.5%). Glucose and insulin responses during the MMTT are shown (Fig. 1A, B). Glucose and insulin AUCs were significantly lower for the MMTT compared with the OGTT (p-values < 0.001). At baseline, participants who later developed diabetes had increased glucose and insulin AUCs/iAUCs in response to the MMTT than non-progressors (Table 1).
Means and SDs for glucose (A) and insulin (B) responses by those who developed diabetes and those who did not. MMTT responses are displayed by and closed black circles with solid lines and open circles with dashed lines for participants who developed type 2 diabetes (+T2D) n = 169(34%) and those who did not (−T2D) n = 332(66%). OGTT responses are displayed similarly with closed and open squares.
MMTT glucose AUC/iAUCs
The HR and 95% confidence intervals from the Cox proportional hazards models assessing the prospective relationship between MMTT glucose AUC/iAUCs on development of diabetes are reported in Fig. 2A and Supplemental Table S1. In unadjusted analyses, glucose AUC180-min (HR: 1.98, 95% CI: 1.67, 2.34, p < 0.0001), AUC240-min (HR: 1.93, 95% CI: 1.62, 2.31, p < 0.0001), and iAUC180-min (HR: 1.43, 95% CI: 1.20, 1.71, p < 0.0001) were associated with an increased risk of diabetes while iAUC240-min (HR: 1.16, 95% CI: 0.98, 1.38, p = 0.09) was not. After further adjustment for covariates (age, sex, body fat [%], M, AIR, SWIA heritage) in three subsequent models AUC180-min (model 4 HR: 1.44, 95% CI: 1.10, 1.88, p = 0.007) and AUC240-min (model 4 HR: 1.41, 95% CI: 1.09, 1.84, p = 0.01) remained associated with increased risk of diabetes. In the final model, after further adjustment for AIR and SWIA, iAUC180-min was no longer associated with increased risk of diabetes (HR: 1.13, 95% CI: 0.91, 1.40, p = 0.27). We also examined glucose and insulin AUC/iAUCs from the OGTT for comparison. These are shown in Supplement Table 3, and as expected, glucose AUC/iAUCs predicted the development of diabetes.
MMTT insulin AUC/iAUCs
The HR and 95% confidence intervals from the Cox proportional hazards models assessing the prospective relationship between MMTT insulin AUC/iAUCs on development of diabetes are reported in Fig. 2B and Supplemental Table S2. In the unadjusted analyses and adjustment for age, sex, and body fat (%), insulin AUC180-min, AUC240-min, iAUC180-min, and iAUC240-min were all independently associated with increased risk of diabetes (p-values < 0.01). However, after adjustment for M (model 3) and further adjustment for AIR and SWIA heritage (model 4) the association between insulin AUC/iAUCs with risk of diabetes was attenuated (p-values > 0.05). As expected, insulin AUC/iAUCs from the OGTT predicted the development of diabetes (Supplemental Table S3).
MMTT peak, rise from fasting, decline from peak
The HR and 95% confidence intervals from the Cox proportional hazards models assessing the prospective relationship between MMTT glucose/insulin peaks, rise from fasting, and decline from peak on development of diabetes are reported in Table 2. Peak glucose was consistently associated with increased risk of diabetes across all four models (model 4 HR: 1.28, 95% CI: 1.03, 1.61, p = 0.03). The association between glucose rise from fasting on risk of diabetes was attenuated after adjustment for AIR and SWIA heritage. Glucose decline from peak was protective against diabetes in models 1 (unadjusted), 2 and 3 but not the final model 4. Insulin peak, rise from fasting and decline from peak were not associated with the risk of diabetes after adjusting for age, sex, and body fat (%) in model 2. Results were similar using the rate of change for rise from fasting and decline from peak to adjust for time (data not shown).
DI as a predictor for MMTT and OGTT
The calculated DI using surrogate measures from the MMTT and OGTT were included in model 2. For MMTT, this measure of DI did not predict diabetes (HR: 0.91; 95% CI: 0.81, 1.03, p = 0.12) but did for OGTT (HR: 0.89; 95% CI: 0.81, 0.99, p = 0.03).
C-Statistic comparisons of MMTT and OGTT
Correlation coefficients between MMTT and OGTT glucose variables are reported in Supplemental Table S4. Due to the moderate to strong correlations between MMTT and OGTT variables C-statistics were computed. C-statistics provide a global measure of model discrimination and were calculated to compare MMTT variables that remained significant after adjustments for age, sex, body fat (%), M, AIR, and SWIA heritage (AUC180-min, AUC240-min, and peak MMTT glucose) to the corresponding OGTT variable, fasting, 60-minute and 120-minute glucose (Supplemental Table S5). The C-statistics for MMTT AUC180-min, AUC240-min, and peak glucoses were similar to OGTT 60-min glucose (AUC180-min 0.72 vs. 0.74, p = 0.31; AUC240-min 0.71 vs. 0.74, p = 0.23; peak 0.71 vs. 0.74, p = 0.07) and OGTT 120-min glucose (AUC180-min 0.72 vs 0.71, p = 0.86; AUC240-min 0.71 vs. 0.72, p = 0.90; peak 0.71 vs. 0.69, p = 0.53). Similarly, MMTT AUC180-min and AUC240-min were similar to the OGTT AUC180-min (AUC180-min 0.72 vs. 0.74, p = 0.15; AUC240-min 0.71 vs. 0.74, p = 0.90) while MMTT peak glucose were also similar to OGTT peak glucose (0.71 vs. 0.73, p = 0.18). The only difference occurred between MMTT glucose AUC180-min and fasting glucose from the OGTT (0.72 vs. 0.69, p = 0.03) indicating the MMTT glucose AUC180-min is slightly more predictive of diabetes compared to fasting glucose from the OGTT.
Discussion
The aim of this analysis was to investigate the utility of an MMTT to predict incident type 2 diabetes. This study, involving a large sample in a well-defined population with detailed reference measurements of important risk factors for diabetes (e.g., insulin action, insulin secretion, and body fat), demonstrated that glucose and insulin responses to a mixed-meal challenge, predicts the development of diabetes even when accounting for these confounders.
Although the glucose and insulin curves were slightly flatter in the MMTT than the OGTT, higher glucose responses in the MMTT were still associated with later development of type 2 diabetes. Clinically, flatter curves reflect the slower carbohydrate absorption expected in a mixed meal test. This may limit the overall variability of the glucose response, but as we have shown the AUCs remain predictive of diabetes in a manner similar to OGTTs. However, in research settings there are many advantages physiologically to MMTTs, including the ability to assess more global physiologic responses including lipid metabolism which, in itself, is an important metabolic pathway for insulin resistance. Across all four models, total glucose AUCs from the MMTT were each independently associated with an increased risk of diabetes even after adjustment for insulin action and secretion. Insulin responses to the MMTT were a consistent predictor of incident diabetes. However, these associations were no longer significant after adjustment for insulin action and secretion.
While other studies have used an MMTT to evaluate glucose and insulin responses in adults with and without type 2 diabetes [3, 9, 29, 30], to our knowledge, ours is the first that has assessed whether responses to an MMTT as predictors of type 2 diabetes. Prior studies that have used an MMTT in adults have found that there is a large amount of inter-individual variability that may be accounted for by genetics, the microbiome, and a plethora of other physiological factors [4, 5, 29, 30]. In studies directly comparing MMTT and OGTTs, glucose responses generate slightly flatter curves in the MMTT compared to the OGTT [29, 30]. MMTTs generate additional and clinically useful data across a range of physiological systems in addition to the risk for type 2 diabetes, including endothelial, renal, and hepatic functioning [3]. Given this, MMTTs are used with increasing frequency in precision nutrition studies. Our data, combined with findings from others, demonstrates that in addition to assessing response to diet interventions, MMTTs also can predict important clinically relevant outcomes.
To date, there is a lack of consensus for designing a standardized MMTT. While this study employed a solid food plus liquid calorie combination, other studies have utilized varying combinations of solid foods [5], solids plus liquids [9, 30], and liquids only [29]. A strength of this study is that the MMTT design used standardized meals that were scaled to be isocaloric to a participant’s weight-maintaining energy needs, providing approximately one-third of their calorie needs for the day within a given meal. Reference measures for insulin action (M) and insulin secretion (AIR) were also included as important covariates in the analyses. MMTT AUC glucose predicted the development of diabetes independent of M and AIR indicating that the mixed macronutrient glucose responses can provide important prognostic indices above gold standard measures of insulin resistance and secretion. Our sample size was also robust; however, it was relatively homogenous as all participants were self-identified as Indigenous Americans. Therefore, it is unknown how results would differ in other populations, but since the underlying pathophysiology of type 2 diabetes is shared, we expect our results to be generalizable. We also recognize that macronutrient content and even such factors as the rate of meal or order of meal ingestion may limit the comparison of results between studies. Although we know the overall macronutrient content of the diets, that the same meals were served for the test, and the calorie load, we do not have the precise meal recipes. We realize this presents challenges in the replication of our data. However, the longitudinal follow-up in our cohort is unique so we feel our data is of value. Lastly, the authors acknowledge that the diagnosis of diabetes is based upon the OGTT rather than diabetes-specific complications such as retinopathy. Future studies may consider evaluating the comparability of the OGTT and MMTT on such clinical endpoints of type 2 diabetes (e.g., retinopathy and/or renal function).
Conclusion
Glucose and insulin responses to body size-adjusted mixed macronutrient challenges predicted the development of type 2 diabetes. Our data indicate that MMTT response. which simulates more “real world” intake can inform health risks beyond the immediate response to dietary interventions.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to previous agreements made between the NIDDK and Indigenous nations who participated in this study. Inquiries may be made to the corresponding author.
References
Centers for Disease Control and Prevention. National Diabetes Statistics Report website. [Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html.
Crandall JP, Knowler WC, Kahn SE, Marrero D, Florez JC, Bray GA, et al. The prevention of type 2 diabetes. Nat Clin Pr Endocrinol Metab. 2008;4:382–93.
Stroeve JHM, van Wietmarschen H, Kremer BHA, van Ommen B, Wopereis S. Phenotypic flexibility as a measure of health: the optimal nutritional stress response test. Genes Nutr. 2015;10:13.
Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, et al. Personalized NUtrition By Prediction Of Glycemic Responses. Cell. 2015;163:1079–94.
Berry SE, Valdes AM, Drew DA, Asnicar F, Mazidi M, Wolf J, et al. Publisher correction: human postprandial responses to food and potential for precision nutrition. Nat Med. 2020;26:1802.
Nuttall FQGM, Wald JL, Ahmed M. Plasma glucose and insulin profiles in normal subjects ingresting diets of varying carbohydrate, fat and protein content. J Am Coll Nutr. 1985;4:437–50.
Nuttall F, Mooradian AD, Gannon MC, Billington CJ, Krezowski P. Effect of protein ingestion on the gluose and insulin response to a standardized oral glucose load. Diabetes Care. 1984;7:465–70.
Denis McGarry J. Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18.
Shankar SS, Vella A, Raymond RH, Staten MA, Calle RA, Bergman RN, et al. Standardized mixed-meal tolerance and arginine stimulation tests provide reproducible and complementary measures of beta-cell function: Results from the foundation for the National Institutes of Health Biomarkers Consortium investigative series. Diabetes Care. 2016;39:1602–13.
Lages M, Barros R, Moreira P, Guarino MP. Metabolic Effects of an Oral Glucose Tolerance Test Compared to the Mixed Meal Tolerance Tests: A Narrative Review. Nutrients. 2022;14:2032.
Department of Health and Human Services. Nutrition for Precision Health 2021 [Available from: https://grants.nih.gov/grants/guide/rfa-files/RFA-RM-21-005.html.
Knowler WC, Bennett PH, Hamman RF, Miller M. Diabetes incidence and prevalence in Pima Indians: a 19-fold greater incidence than in Rochester, Minnesota. Am J Epidemiol. 1978;108:497–505.
Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N. Engl J Med. 1993;329:1988–92.
Paddock E, Hohenadel MG, Piaggi P, Vijayakumar P, Hanson RL, Knowler WC, et al. One-hour and two-hour postload plasma glucose concentrations are comparable predictors of type 2 diabetes mellitus in Southwestern Native Americans. Diabetologia. 2017;60:1704–11.
Shah MH, Piaggi P, Looker HC, Paddock E, Krakoff J, Chang DC. Lower insulin clearance is associated with increased risk of type 2 diabetes in Native Americans. Diabetologia. 2021;64:914–22.
Ferraro R, Boyce VL, Swinburn B, De Gregorio M, Ravussin E. Energy cost of physical activity on a metabolic ward in relationship to obesity. Am J Clin Nutr. 1991;53:1368–71.
Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–7.
Tataranni PA, Ravussin E. Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutr. 1995;62:730–4.
Guo Y, Franks PW, Brookshire T, Antonio Tataranni P. The intra- and inter-instrument reliability of DXA based on ex vivo soft tissue measurements. Obes Res. 2004;12:1925–9.
Bogardus C, Lillioja S, Howard BV, Reaven G, Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. 1984;74:1238–46.
Schwartz MW, Boyko EJ, Kahn SE, Ravussin E, Bogardus C. Reduced insulin secretion: an independent predictor of body weight gain. J Clin Endocrinol Metab. 1995;80:1571–6.
Alferes VR, Alferes VR. Methods of randomization in experimental design. Los Angeles: SAGE; 2012.
Lillioja S, Mott DM, Howard BV, Bennett PH, Yki-Jarvinen H, Freymond D, et al. Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. N. Engl J Med. 1988;318:1217–25.
Ravussin E, Bogardus C. Relationship of genetics, age, and physical fitness to daily energy expenditure and fuel utilization. Am J Clin Nutr. 1989;49:968–75.
Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care. 2009;32:335–41.
Pencina MJ, D’Agostino RB Sr. Evaluating discrimination of risk prediction models: The c statistic. JAMA. 2015;314:1063–4.
Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–23.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the are under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
Wopereis S, Stroeve JHM, Stafleu A, Bakker GCM, Burggraaf J, van Erk MJ, et al. Multi-parameter comparison of a standardized mixed meal tolerance test in healthy and type 2 diabetic subjects: the PhenFlex challenge. Genes Nutr. 2017;12:21.
Caumo A, Bergman RN, Cobelli C. Insulin sensitivity from meal tolerance tests in normal subjects: a minimal model index. J Clin Endocrinol Metab. 2000;85:4396–402.
Acknowledgements
The authors would like to thank the participants who contributed their invaluable data to this project. We would also like to thank the Phoenix Epidemiology and Clinical Research Branch staff for their dedication and assistance to our research participants. Data described in this manuscript, code book, and analytic code will be made available pending application and approval.
Funding
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Author information
Authors and Affiliations
Contributions
C.M.M. and E.J.S. analyzed the data, contributed to discussion, and wrote, reviewed, and edited the manuscript. D.C.C. and J.K. reviewed and edited the manuscript. D.C.C. and J.K. contributed to discussion and reviewed and edited the manuscript. All authors approved the final version of the manuscript. J.K. is the guarantor and therefore takes full responsibility for the work as a whole, including access to data, and the decision to submit and publish the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mitchell, C.M., Stinson, E.J., Chang, D.C. et al. A mixed meal tolerance test predicts onset of type 2 diabetes in Southwestern Indigenous adults. Nutr. Diabetes 14, 50 (2024). https://doi.org/10.1038/s41387-024-00269-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41387-024-00269-3
- Springer Nature Limited