Abstract
Antipsychotic (AP) drugs are efficacious treatments for various psychiatric disorders, but excessive weight gain and subsequent development of metabolic disease remain serious side effects of their use. Increased food intake leads to AP-induced weight gain, but the underlying molecular mechanisms remain unknown. In previous studies, we identified the neuropeptide Agrp and the transcription factor nuclear receptor subfamily 5 group A member 2 (Nr5a2) as significantly upregulated genes in the hypothalamus following AP-induced hyperphagia. While Agrp is expressed specifically in the arcuate nucleus of the hypothalamus and plays a critical role in appetite stimulation, Nr5a2 is expressed in both the CNS and periphery, but its role in food intake behaviors remains unknown. In this study, we investigated the role of hypothalamic Nr5a2 in AP-induced hyperphagia and weight gain. In hypothalamic cell lines, olanzapine treatment resulted in a dose-dependent increase in gene expression of Nr5a2 and Agrp. In mice, the pharmacological inhibition of NR5A2 decreased olanzapine-induced hyperphagia and weight gain, while the knockdown of Nr5a2 in the arcuate nucleus partially reversed olanzapine-induced hyperphagia. Chromatin-immunoprecipitation studies showed for the first time that NR5A2 directly binds to the Agrp promoter region. Lastly, the analysis of single-cell RNA seq data confirms that Nr5a2 and Agrp are co-expressed in a subset of neurons in the arcuate nucleus. In summary, we identify Nr5a2 as a key mechanistic driver of AP-induced food intake. These findings can inform future clinical development of APs that do not activate hyperphagia and weight gain.
Similar content being viewed by others
Introduction
Antipsychotic (AP) medications are efficacious treatments for various psychiatric disorders [1,2,3,4,5,6] but excessive weight gain remains a serious side effect of their use [3, 7, 8]. Approximately 20% of patients treated with a broad range of APs gain clinically significant amounts of weight (>7% of their baseline weight) [9]. Drug safety reviews have shown that the percentage of weight gain varies between individuals and depends on the drug, ranging from ~20–40% for olanzapine (OLZ) and clozapine, and ~10–20% for quetiapine and risperidone [9,10,11,12,13,14,15,16]. APs induce weight gain in human [7, 17,18,19] and rodents [20,21,22,23,24,25,26,27,28,29] by increasing food intake (hyperphagia). However, little is known about the molecular mechanisms by which APs induce hyperphagia. Previous studies have relied on non-specific anti-obesity drugs that suppress basal feeding to reduce AP-induced weight gain (i.e., locaserin [30], orlistat [31], liraglutide [32], nizatidine [33] metformin [34]). While using anti-obesity drugs in combination with APs is clinically beneficial to offset weight gain, they do not shed light on the specific mechanisms underlying AP-induced hyperphagia. Delineating the specific mechanisms driving AP-induced hyperphagia can inform future drug development of highly effective APs without this serious adverse effect and, more broadly, anti-obesity drugs.
In our previous work on AP-induced hyperphagia in C. elegans and mice, we identified gene expression programs altered by AP-induced hyperphagia. The agouti-related peptide Agrp and the transcription factor nuclear receptor subfamily 5 group A member 2 (Nr5a2) genes were significantly upregulated following AP-induced hyperphagia.
The Agrp-expressing neurons in the arcuate nucleus (ARC) of the hypothalamus play a major role in food intake behavior [35,36,37,38,39]. While some studies have reported increased expression of Agrp after AP-treatment [25, 40, 41], the molecular mechanisms regulating the AP-induced expression of this key pro-feeding gene are not well understood [42,43,44]. In contrast, Nr5a2 is broadly expressed throughout the body and has well-described roles in the liver [45, 46], gut [47], and pancreas [48, 49]. Nr5a2 has also been implicated in adipocyte formation [50], intestinal function [47] pancreatic inflammation [48] and expression of pancreatic digestive enzymes [51, 52]. However, little is known about its role in the brain [53, 54]. Within the brain [55], Nr5a2 expression is enriched in the ARC of the hypothalamus [53, 56,57,58] and single-cell analysis has revealed that Nr5a2 expression marks a specific subset of neurons in this region [59]. Our previous studies provided the first insights into the potential involvement of Nr5a2 in AP-induced food intake [26]. In these C.elegans based studies, we determined that Nr5a2 ortholog/nhr-25 mutant strain (nhr-25(ku215)) was resistant to AP-induced hyperphagia [26]. In the current study, we used several mouse models to investigate the role of Nr5a2 in AP-induced food intake and weight gain.
Materials and methods
In vitro studies
Adult mouse hypothalamic cell lines (mHypoA-59, CLU468 cells, Cedarlane) were cultured as described previously [60, 61], and confirmed to be Mycoplasma-free. In brief, cells were grown and maintained in high-glucose, pyruvate-free DMEM supplemented with 10% fetal bovine serum, L-glutamine (Cat. 25030081, Gibco, NY), and 10 u/ml of penicillin and 10 ug/ml of streptomycin (Cat. 15149-122, Gibco) of in a 5% CO2 environment. Cells were treated with OLZ (O0393, TCI Americas) (25–100 µM) for 6 h for mRNA expression analysis of NR5A2 and AGRP. For protein analysis, cells were incubated with 100 µM OLZ for 24 h. For NR5A2 protein nuclear expression studies, cells were co-treated with an NR5A2 antagonist SR1848 (AOB1355, Aobious, Gloucester, MA) for 24 h at 5 µM in DMSO as described previously [62]. For Agrp mRNA expression, cells were incubated with 1–5 µM SR1848 for 6 h, while for AGRP protein expression, cells were incubated with 1 µM of SR1848 for 24 h.
Gene expression
RNA isolation was performed using Trizol (cat # 15596026, Thermo Fisher) and was purified using RNeasy Plus Mini Kit (cat # 774104, Qiagen) using the manufacturer’s recommendations. cDNA was reverse transcribed from 300 ng of RNA using High-Capacity cDNA transcription kit (cat # 4368813, Applied Biosystems). Relative expression was analyzed by qPCR using StepOne Realtime PCR System. Gene expression was calculated after normalization to the housekeeping genes [63] (Pgk1, Hprt1) using the ΔΔCt method. Gene expression was calculated relative to experimental controls. Primer sequences (5’-3’) used to measure gene expression are listed in Table 1.
Western blots
Hypothalamic proteins were isolated using NP-40 with 0.03 M PMSF and cOmplete™, EDTA-free Protease Inhibitor, (Roche 11873580001). Nuclear proteins were isolated from ~6 million HypoA cells treated with either vehicle or 5uM SR1848 for 24 h [62]. Briefly, cells were incubated with a hypotonic solution (10 mM HEPES pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 1 mM DTT, protease inhibitors) for 20 min to isolate cytoplasmic proteins followed by a high salt buffer (20 mM HEPES pH 7.9, 420 mM NaCl, 1.5 mM MgCl2, 1 mM DTT, 25% glycerol, 0.5% Igepal, protease inhibitors) for 50 min to extract nuclear proteins. Proteins were then fractionated in 4–15% Mini PROTEAN TGX acrylamide gels, transferred to PVDF, blocked with 5% BSA, incubated with the primary antibody overnight and secondary antibody for 60 min before detection using ECL (SuperSignal, Thermo Fisher 34580, 34095). Band intensities were quantified using densitometry in ImageLab (Biorad). The following antibodies were used to detect proteins: anti-AGRP (1:100, sc-518077, Santa Cruz Biotechnology), anti-NR5A2 (1:1000, PP-H2325-00, RnD Systems), anti-caveolin (1:1000, 610407, BD Transduction Lab), anti-beta actin (1:2000, 3700, Cell Signaling), anti-histone 3 (1:1000, 4499, Cell Signaling), anti-mouse IgG (1:4000, 115035003, Jackson Immunoresearch), anti-rabbit IgG (1:4000, NA934V, GE Healthcare).
Mice
All protocols were approved by UCSD IACUC. All mice were singly housed in standard cages and acclimated to laboratory conditions (12:12 light–dark, 20–21 °C, 50% humidity) for 7 days before experimentations. Mice were singly housed to accurately measure daily food intake by weighing food in the hopper and accounting for any spillage [64]. All studies were performed in female C57B6/J mice (Jackson, stock # 000664) or Agrp null mice. Agrp−/− (KO) mice were gifted by Dr. Chen Liu of UT Southwestern. Sample sizes were based on previous similar studies [28, 29, 65, 66]. Mice were randomized and all groups were weight-matched prior to any drug treatment or surgeries. Furthermore, the metabolic phenotyping was conducted by one postdoctoral fellow, and no blinding was incorporated.
Olanzapine administration in diet
OLZ was compounded into 45% HFD diet (54 mg/kg = ~6–8 mg/kg) as a convenient dosing strategy [26, 28, 29], and this approach has been used in many other studies investigating AP-induced hyperphagia and weight gain [25, 65, 67,68,69] where HFD feeding potentiates OLZ-induced hyperphagia and weight gain. This dose results in mouse plasma levels (21 ± 5 ng/ml) that are similar to levels observed in humans treated with OLZ (10–50 ng/ml) [65].
Systemic inhibition of NR5A2
Twelve-week old female mice were acclimated to receive intraperitoneal (IP) injections of sterile saline for 3 days before the experiment and then were randomized to receive a 45% high-fat diet (CON, D09092903B, Research Diets) with or without OLZ (54 mg/kg, D16111030). Mice were then further randomized to receive the vehicle solution (VEH, 10% DMSO, 10% Tween 80 in 0.9% NaCl) or the NR5A2 inhibitor (SR1848) at 30 mg/kg daily for 7 days. Food intake and body weight were measured daily. Animals were sacrificed at the end of the study and the hypothalamus was dissected, snap-frozen in liquid nitrogen, and stored at −80 °C until analyses.
Pair feeding studies
Female mice 10–12 weeks of age were divided into three groups: (1) OLZ diet, ad libitum + vehicle, (2) OLZ diet, ad libitum + SR1848 (30 mg/kg), (3) OLZ diet and fed the same average quantity of food that group 2 ate (pair fed) + vehicle. All mice consumed the OLZ diet (54/mg/kg) for the 7 day study.
Hypothalamic inhibition of Nr5a2
Twelve-week old female mice were anesthetized with 5% isoflurane and placed in a stereotactic apparatus (David Kopf Instruments, Model 900HD Motorized Small Animal Stereotaxic). Brain injections were performed in mice under a continuous flow of 2% isoflurane. Correct targeting of the arcuate nucleus of the hypothalamus was confirmed by injection of AAV2-eSyn-EGFP-wpre (Vector Biolabs) using co-ordinates (A–P: −1.58 mm from Bregma; M–L ± 0.25 mm from midline; D–V: −5.8 mm into the skull). Nr5a2 siRNA (SMARTpool: Catalog ID: L-047044-01-0005, Dharmacon, Lafayette, CO) or non-targeting control siRNA (Catalog ID:D-001810-10-05; Dharmacon) (n = 4–5) was delivered bilaterally (200 nl of siRNA) into the ARC using coordinates above. To allow time for diffusion, the injection needle remained immobile for 10 min before removal. Mice were allowed to recover for 7 days before transitioning to CON or OLZ treatment. Food intake was measured daily and body weight every other day for 14 days, at which point many studies have established significant OLZ-induced hyperphagia and weight gain [26, 28, 29, 66, 70]. Animals were sacrificed at the end of the study and the hypothalamus was dissected, snap-frozen in liquid nitrogen, and stored at −80 °C until analyses.
Agrp null studies
Twelve-week old WT and KO female mice were randomized to receive either CON or OLZ (n = 9–17/group). Food intake was measured daily, and body weight was every other day for 12 days. Animals were sacrificed at the end of the study and the hypothalamus was dissected, snap-frozen in liquid nitrogen, and stored at −80 °C until analyses.
Photometry experiments
Female Agrp-ires-cre mice (Jackson Laboratory stock no. 012899), aged 12–20 weeks, received viral infusions (200–300 nl of AAV1-CAG-Flex-GCaMP6s-WPRE-SV40 (Addgene, #100842) into the arcuate nucleus to express GCaMP6s, a potent genetically encoded calcium indicator [71]. Ceramic optical fiber ferrules (400 µm core diameter, 2.5 mm ferrule diameter, NA 0.39, RWD, R-FOC-L400C-39NA) were inserted just dorsal (~300 µm above) to the injection site and fixed to the skull by applying a thin layer of C&B Metabond (Parkell) onto the skull surface, followed by building a cap using dental cement (Ortho-Jet, Lang Dental). Animals were allowed to recover for 2–3 weeks before recordings. Calcium imaging from Agrp neurons was conducted using a commercially available system (FP3002, Neurophotometrics) according to the manufacturer’s instructions. For fiber photometry using GCaMP6s in Agrp neurons, successful targeting of Agrp neurons was tested 2–3 weeks after surgery by fasting the mice for 6–24 h and then refeeding with chow and observing a significant drop in Agrp activity. To measure Agrp responsiveness to OLZ or Veh, mice were injected IP with OLZ (0.5 mg/kg/200 ul volume) or saline based on previous studies [72]. Video of the recording session was collected using a webcam, and was synchronized to the recorded photometry signal. All the recorded traces were aligned relative to the time the mouse was returned to the home cage. Analysis of photometry data was based on previously published methods [73].
Chromatin immunoprecipitation (ChIP)
ChIP experiments were conducted in triplicates using methods previously described in other neuronal cell types [74,75,76]. Briefly, hypothalamic mHypoA-59 cells were grown in 10 cm dishes and at 75–80% confluency and fixed with 1% formaldehyde. Nuclei were isolated before chromatin extraction. Chromatin from ~10 million cells was sheared using a sonication device (Bioruptor Pico, #2013–2019, Diagenode) and optimized to produce ~400 bp fragments. Chromatin was immunoprecipitated using 4 ug of NR5A2 antibody (PP-H2325-00. 5 μg/ChIP, RD Biosystems) [48] and 20 ul of beads without any antibody were used as control samples. Importantly, this antibody has been validated in the Nr5a2 KO [77] and has successfully been used in liver [78, 79] and pancreatic [48] ChIP experiments in mice. After primary and secondary antibody incubation and washes, purified DNA was used in quantitative PCR reactions with primers targeting the promoter of Nr5a2 target gene Prospero Homeobox 1(Prox1) promoter (Prox1-F 5’-CTGTTAACTGTGCCCAGGGAGAGGA-3’, Prox1-R 5’-TGGTTTGACATCTTGGGTGA-3’) [54] as a positive control [54] Prox1_LocA-F: 5’-GTATCTTCACCCGGTTGCTG-3’ Prox-1-LocA-R: 5’-CGATTCATGTAAATAACACC-3’ as a negative control [54], or the Agrp promoter region (Agrp-F 5’-GGGGTCTGGACACCCTATCT-3’, Agrp-R 5’-CACACGTGACTGCTTCCTGT-3’) [80]. Fold enrichment was calculated relative to the no-antibody control samples. Notably, previous published immunohistochemistry and western blot studies have established that Prox 1 is expressed in the rodent hypothalamus [81].
RNAscope
WT female C57B6/J mice were anesthetized with Pentobarbital and then transcardially perfused with 20 ml PBS followed by 20 ml 4% PFA (Sigma). Brains were removed and incubated in 4% PFA overnight at 4 °C. Following cryoprotection in 30% sucrose, 25 μm coronal sections of the brains were collected using a sliding microtome (Leica SM2010R) and stored at −20 °C in a cryoprotectant solution. On the day of RNAScope staining, slices containing the arcuate nucleus were selected for staining and washed 3 times in PBS and then mounted on glass slides (VWR). RNAscope was performed using the RNAscope Multiplex Fluorescent Reagent Kit v2 (ACD, #323100) following the user manual (ACD, USM-323100) with minor modifications. Tissue sections corresponding to ARC from 3 VEH and 3 OLZ mice (1 section per mouse) were hybridized with a mix of two probes; Agrp (ACD, # 400711-C2) + Fos (ACD, # 316921). We used DAPI as a nuclear stain. To assess both tissue RNA integrity and assay procedure, a separate group of sections was incubated with negative probes (data not shown). Images were acquired with the Keyence fluorescence microscope and analyzed with the ImageJ software. The number of Agrp/fos positive cells was manually counted from six sections per mouse, n = 6.
Cell type-specific expression analysis of Nr5a2 and Agrp using single-cell RNA-seq data
The dotplot visualization of Nr5a2 and Agrp expression across 34 neuronal sub-cluster of the ARC was produced using the “explore” function on the Broad Institute single cell portal [82] using Campbell et al. [59] study.
Results
Olanzapine treatment increases the expression of Nr5a2 and Agrp
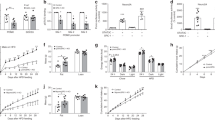
To study the effects of AP on Nr5a2 and Agrp expression, we used OLZ treatment in cell lines and mice. While OLZ is associated with a very high risk for weight gain, it is also regarded as one of the most clinically effective medications [83]. OLZ treatment of hypothalamic cells resulted in a significant dose-dependent upregulation of transcript and protein levels of Nr5a2 (Fig. 1A, B) and Agrp (Fig. 1C, D) compared to vehicle treatment. To assess the effect of OLZ in mice, we measured the expression of Nr5a2 and Agrp in the hypothalamus of mice that were defined as prone (gained 6.3 g body weight) or resistant (gained 1.3 g body weight) to antipsychotic-induced weight gain (AIWG) following OLZ treatment [28]. In addition to the previously noted elevation in the hypothalamic expression of Agrp, we also observed a highly significant elevation of Nr5a2 (Fig. 1E, F) and Agrp (Fig. 1G, H) gene and protein expression in the AIWG-prone compared with the AIWG-resistant mice. In addition, AIWG-prone mice were also hyperphagic compared to AIWG-resistant mice. Thus, the elevated hypothalamic expression of Nr5a2 and Agrp in the AIWG-prone mice further suggests that these genes may play a role in AP-induced hyperphagia and weight gain.
OLZ treatment of hypothalamic cells results in dose-dependent increase in expression of Nr5a2 (A, B) and Agrp (C, D) as determined by qPCR (A, C) and western blotting (B, D). Mice that are highly Prone to Antipsychotic-Induced Weight Gain (AIWG-P) have significantly elevated hypothalamic levels of Nr5a2 (E, F) and Agrp (G, H) gene expression compared with AIWG-Resistant mice (AIWG-R) at both the gene expression (E, G) and protein level (F, H). E Data passed the Shapiro–Wilk test for normality and is expressed as mean ± SEM and was analyzed using either one-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli with a false discovery rate of 0.10 (A, C, n = 3–8 replicates per group) or Student’s t test (B, D–H, n = 3–10 replicates per group), * denotes statistical significance at p < 0.05.
NR5A2 inhibitor treatment reduces OLZ-induced food intake and weight gain
We further investigated the role of Nr5a2 in OLZ-induced hyperphagia in mice using a specific NR5A2 antagonist (SR1848, IP 30 mg/kg daily) [62]. SR1848 inhibits NR5A2 function by triggering translocation of NR5A2 from the nucleus to the cytoplasm, which ultimately abrogates its ability to transduce transcription of its targets [62]. While OLZ treatment resulted in elevated hypothalamic expression of Nr5a2, co-treatment with SR1848 (OLZ + SR) did not impact Nr5a2 expression levels (Fig. 2A). In contrast, co-treatment of OLZ with SR1848 resulted in significantly reduced daily food intake (Fig. 2B) and weight gain (Fig. 2C) compared with OLZ alone over 7 days of treatment. Furthermore, hypothalamic levels of Agrp (Fig. 2D) were significantly reduced by co-treatment, while other appetite regulating neuropeptides Npy and Pomc levels were not significantly changed. As previously shown, SR1848 triggers cytoplasmic translocation of Nr5a2 from the nucleus in epithelial cells [62]. We have replicated this experiment in hypothalamic cells and show that SR1848 treatment results in significantly lower levels of nuclear Nr5a2 protein compared with vehicle treatment (Fig. 2E). To determine whether SR1848 has a direct effect on hypothalamic gene expression, we treated hypothalamic cell lines with SR1848 and measured Agrp gene expression (Fig. 2F). We observed a significant reduction in Agrp expression levels after SR1848 dosing suggesting inhibition of Nr5a2 in the hypothalamus impacts Agrp gene expression. Furthermore, protein levels of Agrp (Fig. 2G) were also significantly decreased after SR1848 treatment in hypothalamic cells.
A Nr5a2 expression, B average daily food intake, C weight gain, D hypothalamic neuropeptide expression in C57BL/6 WT female mice fed either control diet (CON) or OLZ diet and injected with NR5A2 antagonist (SR1848, “SR”, 30 mg/kg) or vehicle (VEH) for 7 days determined by real time RT-qPCR assays. E NR5A2 expression in nuclear fraction of hypothalamic cells lines treated with SR1848 (5 μM for 24 h). F Quantitative PCR determination of Agrp expression in hypothalamic cells treated with SR1848 (1 or 5 μM) for 6 h. G AGRP protein expression in hypothalamic cells treated with SR1848 (1 μM for 24 h). Data passed the Shapiro–Wilk test for normality and are expressed as mean ± SEM and was analyzed using either one-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli with a false discovery rate of 0.10 (A–D, H, I, n = 7–10), or Student’s t test (E–G, n = 4–11 replicates per group), * denotes statistical significance at p < 0.05. ** denotes statistical significance at p < 0.01.
To further support that SR1848 modifies gene expression in the hypothalamus, we treated mice with SR1848 and then extracted the hypothalamus and measured Nr5a2 target gene expressions. Nr5a2 target genes were selected from studies comparing the gene expression profiles of embryonic (E12.5) WT and Nr5a2KO mice [54] or similar in vitro studies with SR1848 [62]. We selected a subgroup of Nr5a2 target genes also expressed in the hypothalamus, according to the Allen brain atlas, which included Prox1, Satb2, Sox4, Ctip2, Jak3, Stat2 Nmi, and Cyp8b1. We found that SR1848 treated mice have decreased expression of Prox1 (a known direct target of Nr5a2) as well as lower expression levels of Satb2, Sox4, Ctip2 and Cyp8b1 and increased expression of Jak3, Stat2 and Nmi (Supplementary Fig. 1A) in line with the previous KO and SR1848 treatment studies [62]. Therefore, the peripheral administration of SR1848 resulted in specific changes in hypothalamic Nr5a2 target genes, further suggesting SR1848 impacts hypothalamic gene expression. Notably, because of the location of the ARC, it is conceivable that SR1848 could impact cells in this area without crossing the blood-brain barrier. However, SR1848 is likely to penetrate the blood-brain barrier based on predictions using a computational software [84].
In a previous study, we did not find any significant effects of OLZ on energy expenditure during the dark and light phases using indirect calorimetry [26]. In addition, SR1848 had no effect on the gene expression of thermogenic genes in the brown adipose tissues (Supplementary Fig. 1B). To determine the contribution of SR1848-induced changes in food intake on body weight gain, we also conducted a pair feeding (PF) study. PF mice were then given the same amount of food that the OLZ + SR1848 treated mice consumed. After a week of treatment, OLZ + PF gained less weight than OLZ + VEH (Fig. 2H), resulting in similar blunting of body weight gain in both OLZ + SR and OLZ + PF groups (Fig. 2I). These PF studies suggest that food intake is the dominant physiological mechanism resulting in a reduction of weight gain in the SR1848-treated mice.
The knockdown of Nr5a2 in the arcuate nucleus partially reversed olanzapine-induced hyperphagia and weight gain
To determine whether OLZ-induced food intake and body weight regulation require the expression of Nr5a2 specifically in the hypothalamus, we used siRNA-mediated knockdown of Nr5a2 expression in the hypothalamus. siRNAs were delivered directly to the hypothalamus by stereotaxic injection (Fig. 3A–C). As expected, Nr5a2 expression was increased by OLZ treatment, and treatment with siRNA targeting Nr5a2 significantly reduced Nr5a2 expression (Fig. 3D). OLZ treatment increased food intake (Fig. 3E) and body weight gain (Fig. 3F, G) which was reversed by hypothalamic Nr5a2 siRNA treatment. Furthermore, gonadal (gWAT) and subcutaneous (sWAT) fat mass were also significantly elevated by OLZ treatment and significantly reduced by Nr5a2 siRNA compared to siRNA control (Fig. 3H).
A–C Bilateral injection of AAV2-eSyn-EGFP (120–150 nl) into the ARC (A–P: −1.58 mm from Bregma; M–L ± 0.25 mm from midline; D–V: −5.8 mm into the skull) (Blue: DAPI, Green: GFP, white dotted lines show ARC boundaries). siRNA-mediated knock down of Nr5a2, delivered by stereotaxic injection to the arcuate nucleus, results in D reduced expression of Nr5a2, E blunted OLZ-induced food intake and F, G reduced OLZ-induced weight gain and body fat (H), Data passed the Shapiro–Wilk test for normality and are expressed as mean ± SEM and were analyzed using either one-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli with a false discovery rate of 0.10 (D–H, n = 4–5), * denotes statistical significance at p < 0.05.
Genetic deletion of Agrp in mice prevented olanzapine-induced hyperphagia and weight gain
Since OLZ treatment increases the expression of Agrp similar to Nr5a2, we used Agrp−/− mice (Fig. 4A–D) to test whether Agrp is necessary for the hyperphagic effect of OLZ. As expected, OLZ treatment of WT mice induced higher food intake (Fig. 4A) and weight gain (Fig. 4B, C) compared with control-treated mice. However, KO mice were resistant to the hyperphagic and weight gain response to OLZ treatment (Fig. 4A–C). While OLZ treatment resulted in elevated hypothalamic transcriptional levels of Nr5a2 in KO mice compared with control-treated KO mice, the expressions of Npy, Pomc and Cart were unchanged (Fig. 4D), suggesting that Nr5a2 may be upstream of Agrp regulation. We also tested whether Agrp neurons increase their activity in response to acute OLZ injection. By expressing a genetically encoded calcium indicator (GCaMP6s) and positioning an optic fiber above the injection site (Supplementary Fig. 2A, B), we recorded their activity in vivo (Supplementary Fig. 2C, D). After the injection of OLZ (IP, 0.5 mg/Kg), we did not observe a difference in the activity of Agrp-expressing neurons compared to the control group (Supplementary Fig. 2E). These results suggest that the effects of chronic OLZ treatment on weight gain and its dependence on Agrp expression do not depend on acute changes in the activity of Agrp neurons. To further support this conclusion, we conducted RNAscope analysis of Fos expression in Agrp-positive neurons following acute OLZ treatment. We observed that only 20% of the Argp neurons co-expressed Fos without significant changes between vehicle and OLZ-treated groups (Supplementary Fig. 2F, G).
Generation and characterization of Agrp−/− mice, A Schematic of the wildtype (WT, +) and knockout (KO, −) alleles. Multiple in-frame (underlined bold) and out-of-frame (underlined) stop codons are inserted into the coding sequences in the exon 5 of Agrp−/− mice. B PCR genotyping of Agrp+/+ and Agrp−/− mice. C Immunostaining of AGRP proteins in the ARC of Agrp+/+ and D Agrp−/− mice. 3V the third ventricle, ARC arcuate nucleus of the hypothalamus. Scale bar is 50 µm. E Food intake, F, G weight gain, H hypothalamic gene expression in WT and KO mice treated with CON or OLZ diets. Data passed the Shapiro–Wilk test for normality and are expressed as mean ± SEM and were analyzed using either one-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli with a false discovery rate of 0.10 (E–H, n = 9–12 per group), * denotes statistical significance at p < 0.05.
Nr5a2 directly binds Agrp promoter
These data led us to hypothesize that Nr5a2 may directly regulate the expression of Agrp by binding to its promoter. To test this, we conducted chromatin immunoprecipitation with Nr5a2 antibodies followed by PCR (ChIP-PCR) in hypothalamic mHypo-A59 cells. In agreement with previous studies in neuronal stem cells [54], we found that Nr5a2 binds the Prospero Homeobox 1(Prox1) promoter (Fig. 5A). We then used primers specific for Agrp promoter region [80] and determined ~2.5-fold enrichment of Nr5a2 binding to the Agrp promoter region over the control sample (Fig. 5B). As a negative internal control, we included a region of the Prox1 promoter where Nr5a2 does not bind (Prox-LocA) and show no enrichment for Nr5a2 binding in this region (Fig. 5B). These ChIP experiments in hypothalamic cells identify Agrp as a direct transcriptional target of the transcription factor Nr5a2. To further explore the relationship between Nr5a2 and Agrp, we analyzed an available single-cell transcriptomic study of the ARC [59] (GSE93374), which identified 34 clusters of molecularly distinct neuronal subtypes. In this dataset, Nr5a2 expression defines one of these subsets (n07.Arx/Nr5a2). We used this dataset to examine the expression of Agrp and Nr5a2 across the ARC sub-neuronal populations (Fig. 5C). This analysis confirmed the previous observations that Nr5a2 is highly expressed in Kiss1 neurons, but importantly also revealed that Nr5a2 is also co-expressed in Agrp populations referred to as n13.Agrp/Gm8773. Therefore, these studies suggest that a specific population of Nr5a2-expressing cells co-express Agrp in the ARC, which play a major role in OLZ-induced hyperphagia and weight gain.
A Agrp promoter region. Chromatin immunoprecipitation with NR5A2 antibodies followed by PCR (ChIP-PCR) in the hypothalamic cell line (mHypo-59A) result in enrichment of binding to the B. Prox1 promoter (positive control), Prox1 LOCA (negative control), Agrp promoter region compared with beads without antibody. Data are expressed as mean ± SEM and were analyzed using Student’s t test (n = 2–4 replicates per group), ** denotes statistical significance at p < 0.01. C Expression of Agrp and Nr5a2 across ARC-ME neuronal populations from GEO dataset GSE93374. Scaling is relative to each gene’s expression across all cells in a given annotation selection, i.e., cells associated with each column label in the dot plot.
Discussion
In these studies, we used several mouse models to investigate the role of Nr5a2 in OLZ-induced food intake and weight gain. We first determined that OLZ treatment resulted in a dose-dependent increase in Nr5a2 and Agrp expression in mouse hypothalamic cells. Furthermore, hypothalamic Nr5a2 expression was highly induced in mice that were particularly prone to AIWG compared with mice that were relatively protected from AIWG. Administration of SR1848, a specific NR5A2 inhibitor, decreased OLZ-induced hyperphagia and weight gain, and the knockdown of Nr5a2 in the ARC partially reversed OLZ-induced hyperphagia. Importantly, Agrp null mice were protected from OLZ-induced hyperphagia and weight gain, despite having elevated hypothalamic Nr5a2 expression, suggesting this transcription factor may regulate Agrp expression. The ChIP-PCR results reported in the current study show, for the first time, that NR5A2 directly binds to the Agrp promoter region and suggest that Nr5a2 directly regulates the expression of this pro-feeding neuropeptide in the hypothalamus. Single-cell RNA-seq studies [59] confirm that Nr5a2 and Agrp are co-expressed in a subset of neurons in the ARC.
Despite the importance of Agrp in the homeostatic control of feeding, the transcriptional regulation of its expression is still poorly understood. Studies have shown that Agrp transcription is regulated by key energy sensors, including peroxisome proliferator-activated receptor gamma coactivator 1-alpha [85], AMP-activated protein kinase or sirtuin 1, and estrogen receptor alpha and signal transducer and activator of transcription 3 [86], forkhead box protein O1 [87], Krüppel-like factor 4 [88]. Our studies discovered a new transcriptional regulator, NR5A2, to this important list of factors that can regulate Agrp expression. Future ChIP-seq studies are warranted to determine the comprehensive transcriptional targets of NR5A2 in Agrp-expressing neurons. Given that Nr5a2 is also expressed in Agrp-negative neuronal subtypes, it will be important to investigate its transcriptional targets in other neuronal populations in the ARC. In addition, Nr5a2 has recently been implicated as playing an important role in the maintenance of neuronal differentiation and identity in the hippocampus. In these studies, deletion of Nr5a2 in the dentate gyrus cells in vivo leads to a reduction of the number of NeuN and calbindin-positive neurons [89]. Similar studies in the hypothalamus will be necessary to reveal if there is a broader function of Nr5a2 in mammalian brain function and plasticity.
To enable transcription factors to bind, chromatin must be in an “open state”. These accessible regions can be determined using Assay for Transposase-Accessible Chromatin combined with sequencing (ATAC-seq). ATAC seq studies from the human prefrontal cortex found enriched motifs for NR5A2 target genes in schizophrenia patients (treated with APs) compared with matched case controls [90]. These studies confirm that APs impact NR5A2 function in the human brain and suggest NR5A2 is an important target for future therapeutic development.
In summary, these studies identify a novel mechanism underlying OLZ-induced hyperphagia. We show that OLZ triggers the transcription of Agrp through NR5A2 in a subset of Agrp-expressing neurons to promote overconsumption and weight gain. These findings can be used to inform future clinical development of APs that do not activate hyperphagia and provide deep insights into the regulation of eating behavior. Importantly, it is critical to mitigate AP-induced weight gain to prevent patient non-compliance [91] and avoid further exacerbating the growing obesity epidemic and the associated increase in the prevalence of metabolic diseases.
References
Moore TJ, Mattison DR. Adult utilization of psychiatric drugs and differences by sex, age, and race. JAMA Intern Med. 2017;177:274–5. https://doi.org/10.1001/jamainternmed.2016.7507.
Painter JT, Owen R, Henderson KL, Bauer MS, Mittal D, Hudson TJ. Analysis of the appropriateness of off-label antipsychotic use for mental health indications in a veteran population. Pharmacotherapy. 2017;37:438–46. https://doi.org/10.1002/phar.1910.
Wofford MR, King DS, Harrell TK. Drug-induced metabolic syndrome. J Clin Hypertens. 2006;8:114–9.
Comer JS, Mojtabai R, Olfson M. National trends in the antipsychotic treatment of psychiatric outpatients with anxiety disorders. Am J Psychiatry. 2011;168:1057–65. https://doi.org/10.1176/appi.ajp.2011.11010087.
Nesvag R, Bramness JG, Handal M, Hartz I, Hjellvik V, Skurtveit S. The incidence, psychiatric co-morbidity and pharmacological treatment of severe mental disorders in children and adolescents. Eur Psychiatry. 2018;49:16–22. https://doi.org/10.1016/j.eurpsy.2017.12.009.
Verdoux H, Tournier M, Begaud B. Antipsychotic prescribing trends: a review of pharmaco-epidemiological studies. Acta Psychiatr Scand. 2010;121:4–10. https://doi.org/10.1111/j.1600-0447.2009.01425.x.
Jensen GL. Drug-induced hyperphagia: what can we learn from psychiatric medications? J Parenter Enter Nutr. 2008;32:578–81. https://doi.org/10.1177/0148607108321708.
Verhaegen AA, Van Gaal LF. Drug-induced obesity and its metabolic consequences: a review with a focus on mechanisms and possible therapeutic options. J Endocrinol Invest. 2017;40:1165–74. https://doi.org/10.1007/s40618-017-0719-6.
Bak M, Fransen A, Janssen J, van Os J, Drukker M. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS ONE. 2014;9:e94112. https://doi.org/10.1371/journal.pone.0094112.
Asenjo Lobos C, Komossa K, Rummel-Kluge C, Hunger H, Schmid F, Schwarz S, et al. Clozapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010:CD006633. https://doi.org/10.1002/14651858.CD006633.pub2.
Komossa K, Rummel-Kluge C, Hunger H, Schmid F, Schwarz S, Duggan L, et al. Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010:CD006654. https://doi.org/10.1002/14651858.CD006654.pub2.
Komossa K, Rummel-Kluge C, Schmid F, Hunger H, Schwarz S, Srisurapanont M, et al. Quetiapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010:CD006625. https://doi.org/10.1002/14651858.CD006625.pub2.
Komossa K, Rummel-Kluge C, Schwarz S, Schmid F, Hunger H, Kissling W, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011:CD006626. https://doi.org/10.1002/14651858.CD006626.pub2.
Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–23. https://doi.org/10.1056/NEJMoa051688.
Musil R, Obermeier M, Russ P, Hamerle M. Weight gain and antipsychotics: a drug safety review. Expert Opin Drug Saf. 2015;14:73–96. https://doi.org/10.1517/14740338.2015.974549.
Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(Suppl 1):1–93.
Fountaine RJ, Taylor AE, Mancuso JP, Greenway FL, Byerley LO, Smith SR, et al. Increased food intake and energy expenditure following administration of olanzapine to healthy men. Obesity. 2010;18:1646–51. https://doi.org/10.1038/oby.2010.6.
Gothelf D, Falk B, Singer P, Kairi M, Phillip M, Zigel L, et al. Weight gain associated with increased food intake and low habitual activity levels in male adolescent schizophrenic inpatients treated with olanzapine. Am J Psychiatry. 2002;159:1055–7. https://doi.org/10.1176/appi.ajp.159.6.1055.
Kluge M, Schuld A, Himmerich H, Dalal M, Schacht A, Wehmeier PM, et al. Clozapine and olanzapine are associated with food craving and binge eating: results from a randomized double-blind study. J Clin Psychopharmacol. 2007;27:662–6. https://doi.org/10.1097/jcp.0b013e31815a8872.
Cooper GD, Goudie AJ, Halford JC. Acute effects of olanzapine on behavioural expression including the behavioural satiety sequence in female rats. J Psychopharmacol. 2010;24:1069–78. https://doi.org/10.1177/0269881109102543.
Davoodi N, Kalinichev M, Korneev SA, Clifton PG. Hyperphagia and increased meal size are responsible for weight gain in rats treated sub-chronically with olanzapine. Psychopharmacology. 2009;203:693–702. https://doi.org/10.1007/s00213-008-1415-1.
Minet-Ringuet J, Even PC, Guesdon B, Tome D, de Beaurepaire R. Effects of chronic neuroleptic treatments on nutrient selection, body weight, and body composition in the male rat under dietary self-selection. Behav Brain Res. 2005;163:204–11. https://doi.org/10.1016/j.bbr.2005.05.004.
Thornton-Jones Z, Neill JC, Reynolds GP. The atypical antipsychotic olanzapine enhances ingestive behaviour in the rat: a preliminary study. J Psychopharmacol. 2002;16:35–7. https://doi.org/10.1177/026988110201600111.
Weston-Green K, Huang XF, Deng C. Olanzapine treatment and metabolic dysfunction: a dose response study in female Sprague Dawley rats. Behav Brain Res. 2011;217:337–46. https://doi.org/10.1016/j.bbr.2010.10.039.
Zhang Q, He M, Deng C, Wang H, Lian J, Huang XF. Hypothalamic ghrelin signalling mediates olanzapine-induced hyperphagia and weight gain in female rats. Int J Neuropsychopharmacol. 2014;17:807–18. https://doi.org/10.1017/S1461145713001697.
Perez-Gomez A, Carretero M, Weber N, Peterka V, To A, Titova V, et al. A phenotypic Caenorhabditis elegans screen identifies a selective suppressor of antipsychotic-induced hyperphagia. Nat Commun. 2018;9:5272. https://doi.org/10.1038/s41467-018-07684-y.
Wei H, Zapata RC, Lopez-Valencia M, Aslanoglou D, Farino ZJ, Benner V, et al. Dopamine D2 receptor signaling modulates pancreatic beta cell circadian rhythms. Psychoneuroendocrinology. 2020;113:104551. https://doi.org/10.1016/j.psyneuen.2019.104551.
Zapata RC, Osborn O. Susceptibility of male wild type mouse strains to antipsychotic-induced weight gain. Physiol Behav. 2020;220:112859. https://doi.org/10.1016/j.physbeh.2020.112859.
Zapata RC, Rosenthal SB, Fisch K, Dao K, Jain M, Osborn O. Metabolomic profiles associated with a mouse model of antipsychotic-induced food intake and weight gain. Sci Rep. 2020;10:18581. https://doi.org/10.1038/s41598-020-75624-2.
Lord CC, Wyler SC, Wan R, Castorena CM, Ahmed N, Mathew D, et al. The atypical antipsychotic olanzapine causes weight gain by targeting serotonin receptor 2C. J Clin Invest. 2017;127:3402–6. https://doi.org/10.1172/JCI93362.
Hilger E, Quiner S, Ginzel I, Walter H, Saria L, Barnas C. The effect of orlistat on plasma levels of psychotropic drugs in patients with long-term psychopharmacotherapy. J Clin Psychopharmacol. 2002;22:68–70.
Fink-Jensen A, Correll CU, Vilsboll T. Liraglutide for the treatment of antipsychotic drug-induced weight gain-reply. JAMA Psychiatry. 2017;74:1173–4. https://doi.org/10.1001/jamapsychiatry.2017.2702.
Sacchetti E, Guarneri L, Bravi D. H(2) antagonist nizatidine may control olanzapine-associated weight gain in schizophrenic patients. Biol Psychiatry. 2000;48:167–8.
Baptista T, Martínez J, Lacruz A, Rangel N, Beaulieu S, Serrano A, et al. Metformin for prevention of weight gain and insulin resistance with olanzapine: a double-blind placebo-controlled trial. Can J Psychiatry. 2006;51:192–6. https://doi.org/10.1177/070674370605100310.
Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci USA. 1998;95:15043–8.
Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271–2. https://doi.org/10.1038/1082.
Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–5. https://doi.org/10.1038/nn.2739.
Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–8. https://doi.org/10.1172/JCI46229.
Stanley BG, Kyrkouli SE, Lampert S, Leibowitz SF. Neuropeptide Y chronically injected into the hypothalamus: a powerful neurochemical inducer of hyperphagia and obesity. Peptides. 1986;7:1189–92.
Fernø J, Varela L, Skrede S, Vázquez MJ, Nogueiras R, Diéguez C, et al. Olanzapine-induced hyperphagia and weight gain associate with orexigenic hypothalamic neuropeptide signaling without concomitant AMPK phosphorylation. PLoS ONE. 2011;6:e20571. https://doi.org/10.1371/journal.pone.0020571.
Martins PJ, Haas M, Obici S. Central nervous system delivery of the antipsychotic olanzapine induces hepatic insulin resistance. Diabetes. 2010;59:2418–25. https://doi.org/10.2337/db10-0449.
Miller CL. On the mechanism of action of antipsychotic drugs: a chemical reaction not receptor blockade. Curr Drug Discov Technol. 2013;10:195–208.
Panariello F, Polsinelli G, Borlido C, Monda M, De Luca V. The role of leptin in antipsychotic-induced weight gain: genetic and non-genetic factors. J Obes. 2012;2012:572848. https://doi.org/10.1155/2012/572848.
Shams TA, Muller DJ. Antipsychotic induced weight gain: genetics, epigenetics, and biomarkers reviewed. Curr Psychiatry Rep. 2014;16:473. https://doi.org/10.1007/s11920-014-0473-9.
Fayard E, Auwerx J, Schoonjans K. LRH-1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol. 2004;14:250–60. https://doi.org/10.1016/j.tcb.2004.03.008.
Oosterveer MH, Mataki C, Yamamoto H, Harach T, Moullan N, van Dijk TH, et al. LRH-1-dependent glucose sensing determines intermediary metabolism in liver. J Clin Invest. 2012;122:2817–26. https://doi.org/10.1172/JCI62368.
Fernandez-Marcos PJ, Auwerx J, Schoonjans K. Emerging actions of the nuclear receptor LRH-1 in the gut. Biochim Biophys Acta. 2011;1812:947–55. https://doi.org/10.1016/j.bbadis.2010.12.010.
Cobo I, Martinelli P, Flández M, Bakiri L, Zhang M, Carrillo-de-Santa-Pau E, et al. Transcriptional regulation by NR5A2 links differentiation and inflammation in the pancreas. Nature. 2018;554:533–7. https://doi.org/10.1038/nature25751.
Cobo-Vuilleumier N, Lorenzo PI, Rodríguez NG, Herrera Gómez IG, Fuente-Martin E, López-Noriega L, et al. LRH-1 agonism favours an immune-islet dialogue which protects against diabetes mellitus. Nat Commun. 2018;9:1488. https://doi.org/10.1038/s41467-018-03943-0.
Mrosek N, Meissburger B, Mataki C, Roeder E, Ukropec J, Klimes I, et al. Transcriptional regulation of adipocyte formation by the liver receptor homologue 1 (Lrh1)-Small hetero-dimerization partner (Shp) network. Mol Metab. 2013;2:314–23. https://doi.org/10.1016/j.molmet.2013.03.003.
Lee YK, Schmidt DR, Cummins CL, Choi M, Peng L, Zhang Y, et al. Liver receptor homolog-1 regulates bile acid homeostasis but is not essential for feedback regulation of bile acid synthesis. Mol Endocrinol. 2008;22:1345–56. https://doi.org/10.1210/me.2007-0565.
Sablin EP, Krylova IN, Fletterick RJ, Ingraham HA. Structural basis for ligand-independent activation of the orphan nuclear receptor LRH-1. Mol Cell. 2003;11:1575–85.
Higashiyama H, Kinoshita M, Asano S. Expression profiling of liver receptor homologue 1 (LRH-1) in mouse tissues using tissue microarray. J Mol Histol. 2007;38:45–52. https://doi.org/10.1007/s10735-007-9077-6.
Stergiopoulos A, Politis PK. Nuclear receptor NR5A2 controls neural stem cell fate decisions during development. Nat Commun. 2016;7:12230. https://doi.org/10.1038/ncomms12230.
Grgurevic N, Tobet S, Majdic G. Widespread expression of liver receptor homolog 1 in mouse brain. Neuro Endocrinol Lett. 2005;26:541–7.
Atkin SD, Owen BM, Bookout AL, Cravo RM, Lee C, Elias CF, et al. Nuclear receptor LRH-1 induces the reproductive neuropeptide kisspeptin in the hypothalamus. Mol Endocrinol. 2013;27:598–605. https://doi.org/10.1210/me.2012-1371.
Gofflot F, Chartoire N, Vasseur L, Heikkinen S, Dembele D, Le Merrer J, et al. Systematic gene expression mapping clusters nuclear receptors according to their function in the brain. Cell. 2007;131:405–18. https://doi.org/10.1016/j.cell.2007.09.012.
Zhou S, Zang S, Hu Y, Shen Y, Li H, Chen W, et al. Transcriptome-scale spatial gene expression in rat arcuate nucleus during puberty. Cell Biosci. 2022;12:8. https://doi.org/10.1186/s13578-022-00745-2.
Campbell JN, Macosko EZ, Fenselau H, Pers TH, Lyubetskaya A, Tenen D, et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci. 2017;20:484–96. https://doi.org/10.1038/nn.4495.
Chen X, Yu Y, Zheng P, Jin T, He M, Zheng M, et al. Olanzapine increases AMPK-NPY orexigenic signaling by disrupting H1R-GHSR1a interaction in the hypothalamic neurons of mice. Psychoneuroendocrinology. 2020;114:104594. https://doi.org/10.1016/j.psyneuen.2020.104594.
Dalvi PS, Nazarians-Armavil A, Tung S, Belsham DD. Immortalized neurons for the study of hypothalamic function. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1030–52. https://doi.org/10.1152/ajpregu.00649.2010.
Corzo CA, Mari Y, Chang MR, Khan T, Kuruvilla D, Nuhant P, et al. Antiproliferation activity of a small molecule repressor of liver receptor homolog 1. Mol Pharm. 2015;87:296–304. https://doi.org/10.1124/mol.114.095554.
Li B, Matter EK, Hoppert HT, Grayson BE, Seeley RJ, Sandoval DA. Identification of optimal reference genes for RT-qPCR in the rat hypothalamus and intestine for the study of obesity. Int J Obes. 2014;38:192–7. https://doi.org/10.1038/ijo.2013.86.
Cameron KM, Speakman JR. The extent and function of ‘food grinding’ in the laboratory mouse (Mus musculus). Lab Anim. 2010;44:298–304. https://doi.org/10.1258/la.2010.010002.
Morgan AP, Crowley JJ, Nonneman RJ, Quackenbush CR, Miller CN, Ryan AK, et al. The antipsychotic olanzapine interacts with the gut microbiome to cause weight gain in mouse. PLoS ONE. 2014;9:e115225. https://doi.org/10.1371/journal.pone.0115225.
Zapata RC, Chaudry BS, Valencia ML, Zhang D, Ochsner SA, McKenna NJ, et al. Conserved immunomodulatory transcriptional networks underlie antipsychotic-induced weight gain. Transl Psychiatry. 2021;11:405. https://doi.org/10.1038/s41398-021-01528-y.
Stefanidis A, Verty AN, Allen AM, Owens NC, Cowley MA, Oldfield BJ. The role of thermogenesis in antipsychotic drug-induced weight gain. Obesity. 2009;17:16–24. https://doi.org/10.1038/oby.2008.468.
Huang XF, Han M, Huang X, Zavitsanou K, Deng C. Olanzapine differentially affects 5-HT2Aand2C receptor mRNA expression in the rat brain. Behav Brain Res. 2006;171:355–62. https://doi.org/10.1016/j.bbr.2006.03.040.
Zhang Q, Lian J, He M, Deng C, Wang H, Huang XF. Olanzapine reduced brown adipose tissue thermogenesis and locomotor activity in female rats. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:172–80. https://doi.org/10.1016/j.pnpbp.2014.02.003.
Pai N, Deng C, Vella SL, Castle D, Huang XF. Are there different neural mechanisms responsible for three stages of weight gain development in anti-psychotic therapy: temporally based hypothesis. Asian J Psychiatr. 2012;5:315–8. https://doi.org/10.1016/j.ajp.2012.05.005.
Dana H, Sun Y, Mohar B, Hulse BK, Kerlin AM, Hasseman JP, et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat Methods. 2019;16:649–57. https://doi.org/10.1038/s41592-019-0435-6.
Kaur G, Kulkarni SK. Studies on modulation of feeding behavior by atypical antipsychotics in female mice. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:277–85. https://doi.org/10.1016/s0278-5846(01)00266-4.
Zalocusky KA, Ramakrishnan C, Lerner TN, Davidson TJ, Knutson B, Deisseroth K. Nucleus accumbens D2R cells signal prior outcomes and control risky decision-making. Nature. 2016;531:642–6. https://doi.org/10.1038/nature17400.
Fields J, Swinton M, Montilla-Perez P, Ricciardelli E, Telese F. The Cannabinoid receptor agonist, WIN, suppresses the activation of proinflammatory genes induced by interleukin 1 beta in human astrocytes. Cannabis Cannabinoid Res. 2020. https://doi.org/10.1089/can.2020.0128.
Ma Q, Telese F. Genome-wide epigenetic analysis of MEF2A and MEF2C transcription factors in mouse cortical neurons. Commun Integr Biol. 2015;8:e1087624. https://doi.org/10.1080/19420889.2015.1087624.
Wang J, Telese F, Tan Y, Li W, Jin C, He X, et al. LSD1n is an H4K20 demethylase regulating memory formation via transcriptional elongation control. Nat Neurosci. 2015;18:1256–64. https://doi.org/10.1038/nn.4069.
Miranda DA, Krause WC, Cazenave-Gassiot A, Suzawa M, Escusa H, Foo JC, et al. LRH-1 regulates hepatic lipid homeostasis and maintains arachidonoyl phospholipid pools critical for phospholipid diversity. JCI Insight. 2018;3:e96151. https://doi.org/10.1172/jci.insight.96151.
Chong HK, Biesinger J, Seo YK, Xie X, Osborne TF. Genome-wide analysis of hepatic LRH-1 reveals a promoter binding preference and suggests a role in regulating genes of lipid metabolism in concert with FXR. BMC Genomics. 2012;13:51. https://doi.org/10.1186/1471-2164-13-51.
Milona A, Massafra V, Vos H, Naik J, Artigas N, Paterson H, et al. Steroidogenic control of liver metabolism through a nuclear receptor-network. Mol Metab. 2019;30:221–9. https://doi.org/10.1016/j.molmet.2019.09.007.
Lee B, Kim SG, Kim J, Choi KY, Lee S, Lee SK, et al. Brain-specific homeobox factor as a target selector for glucocorticoid receptor in energy balance. Mol Cell Biol. 2013;33:2650–8. https://doi.org/10.1128/MCB.00094-13.
Galeeva A, Treuter E, Tomarev S, Pelto-Huikko M. A prospero-related homeobox gene Prox-1 is expressed during postnatal brain development as well as in the adult rodent brain. Neuroscience. 2007;146:604–16. https://doi.org/10.1016/j.neuroscience.2007.02.002.
Campbell JN, Macosko EZ, Fenselau H, Pers TH, Lyubetskaya A, Tenen D, et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci 2017;20:484–96.
Manschreck TC, Boshes RA. The CATIE schizophrenia trial: results, impact, controversy. Harv Rev Psychiatry. 2007;15:245–58. https://doi.org/10.1080/10673220701679838.
Liu H, Wang L, Lv M, Pei R, Li P, Pei Z, et al. AlzPlatform: an Alzheimer’s disease domain-specific chemogenomics knowledgebase for polypharmacology and target identification research. J Chem Inf Model. 2014;54:1050–60. https://doi.org/10.1021/ci500004h.
Gill JF, Delezie J, Santos G, Handschin C. PGC-1alpha expression in murine AgRP neurons regulates food intake and energy balance. Mol Metab. 2016;5:580–8. https://doi.org/10.1016/j.molmet.2016.05.008.
Benedusi V, Della Torre S, Mitro N, Caruso D, Oberto A, Tronel C, et al. Liver ERalpha regulates AgRP neuronal activity in the arcuate nucleus of female mice. Sci Rep. 2017;7:1194. https://doi.org/10.1038/s41598-017-01393-0.
Kitamura T, Feng Y, Kitamura YI, Chua SC Jr, Xu AW, Barsh GS, et al. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–40. https://doi.org/10.1038/nm1392.
Ilnytska O, Stütz AM, Park-York M, York DA, Ribnicky DM, Zuberi A, et al. Molecular mechanisms for activation of the agouti-related protein and stimulation of appetite. Diabetes. 2011;60:97–106. https://doi.org/10.2337/db10-0172.
Tsampoula M, Tarampoulous I, Antoniadou I, Koutmani Y, Gkikas D, Vekrellis K, et al. Nuclear receptor NR5A2 promotes neuronal identity in the adult hippocampus. Mol Neurobiol. 2021;58:1952–62. https://doi.org/10.1007/s12035-020-02222-8.
Bryois J, Garrett ME, Song L, Safi A, Giusti-Rodriguez P, Johnson GD, et al. Evaluation of chromatin accessibility in prefrontal cortex of individuals with schizophrenia. Nat Commun. 2018;9:3121. https://doi.org/10.1038/s41467-018-05379-y.
Tschoner A, Engl J, Laimer M, Kaser S, Rettenbacher M, Fleischhacker WW, et al. Metabolic side effects of antipsychotic medication. Int J Clin Pr. 2007;61:1356–70. https://doi.org/10.1111/j.1742-1241.2007.01416.x.
Acknowledgements
This study was accomplished through the support by grants including the National Institutes of Health (NIH) grant R01DK117872 (OO), NIH grant P30 DK063491 (Diabetes Research Center, UCSD) (FT and OO), NIH grants DK130892 (CL) and DK114036 (CL), AG066821 (SMC), American Heart Association Post-doctoral Fellowship 18POST33960457 (ZZ) and Larry L. Hillblom Foundation Postdoctoral Fellowship 2019-D-007-FEL (RCZ).
Author information
Authors and Affiliations
Contributions
RCZ, ZZ, AP, AL and OO performed in vivo experiments. RCZ, DZ, PMP, AL and AN performed laboratory analyses. BX and CL provided the Agrp null mice. SMC and FT provided intellectual input and oversight of technical expertise. RCZ, OO, AL and FT analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zapata, R.C., Zhang, D., Libster, A. et al. Nuclear receptor 5A2 regulation of Agrp underlies olanzapine-induced hyperphagia. Mol Psychiatry 28, 1857–1867 (2023). https://doi.org/10.1038/s41380-023-01981-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-01981-9
- Springer Nature Limited
This article is cited by
-
Psychosis and autism spectrum disorder: a special issue of Molecular Psychiatry
Molecular Psychiatry (2023)