Abstract
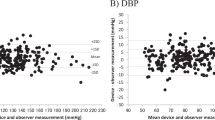
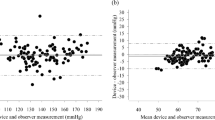
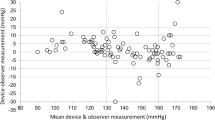
The present study aimed to evaluate the accuracy of the Omron HBP-9031C automated oscillometric upper-arm blood pressure (BP) measurement device for professional office BP monitoring, according to the ANSI/AAMI/ISO 81060-2:2013 protocol (ANSI/AAMI/ISO) in the general population. The device was assessed by using it on 85 participants, who fulfilled our inclusion criteria involving the ranges of arm circumference and systolic and diastolic BP. The validation and data analysis were performed as per the protocol. In the ANSI/AAMI/ISO 81060-2:2013 validation procedure (criterion 1), the mean ± SD of the differences between the test device and reference BP was 1 ± 8/−2 ± 6 mmHg (systolic/diastolic). The mean differences between the two observers and the Omron HBP-9031C were 1 ± 7 mmHg for systolic BP and −2 ± 6 mmHg for diastolic BP, according to criterion 2. The two ANSI/AAMI/ISO criteria were fulfilled. The OMRON HBP-9031C professional BP monitor fulfilled the requirements of the ANSI/AAMI/ISO validation standard and can be recommended for clinical use.

Similar content being viewed by others
References
Association for the Advancement of Medical Instrumentation American National Standard. ANSI/AAMI/ISO 81060-2:2013 non-invasive sphygmomanometers - part 2: clinical investigation of automated measurement type. 2013. http://my.aami.org/aamiresources/previewfiles/8106002_1306_preview.pdf#search=%27American+National+Standard.+ANSI%2FAAMI%2FISO+810602%3A2013+Noninvasive%27. Accessed 2 Jul 2019.
Stergiou GS, Alpert B, Mieke S, Asmar R, Atkins N, Eckert S, et al. A universal standard for the validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. J Hypertens. 2018;36:472–8.
Acknowledgements
We would like to thank Editage (www.editage.com) for their English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saito, K., Hishiki, Y. & Takahashi, H. Validation of the Omron HBP-9031C professional office blood pressure monitor in the general population according to the ANSI/AAMI/ISO 81060-2:2013 protocol. J Hum Hypertens 34, 735–738 (2020). https://doi.org/10.1038/s41371-020-0301-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-020-0301-0
- Springer Nature Limited