Abstract
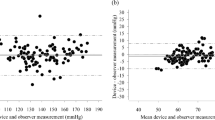
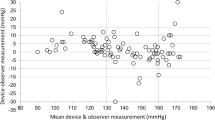
The performance of Omron HEM-9200T for monitoring blood pressure (BP) in the upper arm was validated in accordance with the American National Standards Institute/Association for the Advancement of Medical Instrumentation/International Organization for Standardization (ANSI/AAMI/ISO) 81060-2:2013 protocol. The device was assessed by using it on 87 participants who fulfilled the inclusion criteria involving the ranges of arm circumference and systolic and diastolic BP provided by the protocol. Validation and data analysis were performed according to the protocol. In the ANSI/AAMI/ISO 81060-2:2013 validation procedure (criterion 1), the mean ± standard deviation of the differences between the test device and reference BP was −0.1 ± 5.06/1.2 ± 5.8 mmHg (systolic/diastolic). The mean differences between the two observers and Omron HEN-9200T were −0.1 ± 3.82 mmHg for systolic BP and 1.2 ± 5.34 mmHg for diastolic BP, fulfilling criterion 2 with an SD of ≤6.91 for SBP and ≤6.87 for DBP. These two ANSI/AAMI/ISO criteria were fulfilled.
The Omron HEM-9200T BP monitor fulfilled the requirements of the ANSI/AAMI/ISO validation standard and can be recommended for BP measurements at home in the general population.

Similar content being viewed by others
References
Association for the Advancement of Medical Instrumentation American National Standard. ANSI/AAMI/ISO 81060-2:2013 non-invasive sphygmomanometers – part 2: clinical investigation of automated measurement type. http://my.aami.org/aamiresources/previewfiles/8106002_1306_preview.pdf#search=%27American+National+Standard.+ANSI%2FAAMI%2FISO+810602%3A2013+Noninvasive%27. Accessed 15 Sep 2020.
Stergiou GS, Alpert B, Mieke S, Asmar R, Atkins N, Eckert S, et al. A universal standard for the validation of blood pressure measuring devices: association for the advancement of medical instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. Hypertension. 2018;71:368–74.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KS and NY are employed by Omron Healthcare Co., Ltd, the manufacturer of Omron HEM-9200T. The authors report no other conflicts of interest in this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Takahashi, H., Saito, K. & Yakura, N. Validation of Omron HEM-9200T, a home blood pressure monitoring device for the upper arm, according to the American National Standards Institute/Association for the Advancement of Medical Instrumentation/International Organization for Standardization 81060-2:2013 protocol. J Hum Hypertens 36, 416–419 (2022). https://doi.org/10.1038/s41371-021-00536-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-021-00536-1
- Springer Nature Limited
This article is cited by
-
A Randomized Controlled Trial to Improve Unmet Social Needs and Clinical Outcomes Among Adults with Diabetes
Journal of General Internal Medicine (2024)