Abstract
Background and objectives
Gut dysbiosis that resulted from the alteration between host-microbe interaction might worsen obesity-induced systemic inflammation. Gut microbiota manipulation by supplementation of prebiotic inulin may reverse metabolic abnormalities and improve obesity. This study aimed to determine whether inulin supplementation improved intestinal microbiota and microbial functional pathways in children with obesity.
Methods
Children with obesity whose BMI above median + 2SDs were recruited to a randomized, double-blinded placebo-controlled study. The participants aged 7–15 years were assigned to inulin supplement extracted from Thai Jerusalem artichoke (intervention), maltodextrin (placebo), and dietary fiber advice groups. All participants received similar monthly conventional advice and follow-up for 6 months. Fecal samples were collected for gut microbiome analysis using 16S rRNA sequencing. Phylogenetic Investigation of Communities by Reconstruction of Unobserved States was performed to infer microbial functional pathways.
Results
One hundred and forty-three children with available taxonomic and functional pathway abundance profiles were evaluated. A significant increase in alpha-diversity was observed in the inulin group. Inulin supplementation substantially enhanced Bifidobacterium, Blautia, Megasphaera, and several butyrate-producing bacteria, including Agathobacter, Eubacterium coprostanoligenes, and Subdoligranulum, compared to the other groups. The inulin group showed a significant difference in functional pathways of proteasome and riboflavin metabolism. These changes correlated with clinical and metabolic outcomes exclusively in the inulin group.
Conclusions
Inulin supplementation significantly promoted gut bacterial diversity and improved gut microbiota dysbiosis in children with obesity. The modulation of functional pathways by inulin suggests its potential to establish beneficial interactions between the gut microbiota and host physiology. Inulin supplementation could be a strategic treatment to restore the balance of intestinal microbiota and regulate their functions in childhood obesity.
Similar content being viewed by others
Introduction
Obesity is a global health concern and the number of children as well as adolescents with overweight or obesity is dramatically high [1]. These children are prone to develop several co-morbidities such as dyslipidemia, non-alcoholic fatty liver disease, and type 2 diabetes mellitus [2].
Recent evidence has suggested that the gut microbiota is involved in energy regulation along with an inflammatory status [3]. The imbalance or dysbiosis of the gut microbiota should be recognized as environmental factors that influence the pathophysiological mechanisms underlying obesity [4]. Many studies in humans have shown differences in the composition and diversity of gut microbiota between individuals with and without obesity [5,6,7]. Obesity in humans has already been associated with the low relative abundance of Bacteroidetes and Actinobacteria, but high relative abundance of Firmicutes [5, 8]. Therefore, consumption of prebiotics, utilized by gut microbiota to regulate host physiology, could be a strategic treatment, and reverses metabolic abnormalities in childhood obesity [8]. Prebiotic supplementation promoted an increase in Bifidobacterium and other beneficial microbes related to their positive effects on host health via microbiota-derived bioactive molecules, such as short chain fatty acids (SCFAs), leading to decreased body weight and adiposity partly through the attenuation of metabolic derangement [9]. There have been studies regarding the effects of prebiotics on gut microbiota in adults with obesity; however, the outcomes were inconsistent [10, 11]. Furthermore, effects of prebiotics on gut microbiota may differ between adult and pediatric populations. Unfortunately, there have been very few interventional studies about prebiotics in children with obesity. For example, researchers found oligofructose-enriched inulin (OI) increased Bifidobacterium by using PCR technique, but not by 16S rRNA sequencing compared to the placebo [12]. There was a study about positive effects of OI on the change in satiety hormones and energy intake [13]. Another study in children and adolescents with obesity showed that oligofructose supplementation for 12 weeks had no effect on body weight in this population [14]. These suggest that studies of the impact of prebiotic supplementation on childhood obesity were very limited and remain inconclusive. Therefore, we aimed to evaluate the effects of prebiotic supplementation as inulin on gut microbiota and microbial functional pathways in children with obesity. Moreover, the relationships between the change in gut microbiota and the changes in clinical or metabolic features were assessed.
Methods
Study participants
This study was a randomized double-blinded placebo-controlled trial conducted from August 2017 to July 2020 at the King Chulalongkorn Memorial Hospital (KCMH), Thailand. The detailed protocol was previously described [15]. This trial was registered at clinicaltrials.gov as NCT03968003. Children with obesity aged 7–15 years whose body mass index (BMI) were above median plus 2 standard deviations (SDs) from the WHO growth reference [1] were enrolled from the KCMH and the social media. All the children who met the inclusion criteria were recruited as mentioned in the previously published study [15].
Study design
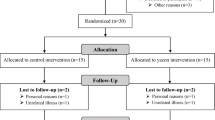
The detailed study design has been mentioned elsewhere [15]. In brief, one hundred and sixty-five participants were randomly assigned to three groups. In the inulin group, participants consumed the extracted inulin powder from Thai Jerusalem artichoke, using our patented technique, approximately 30 min before dinner each day. Similarly, the placebo group received isocaloric maltodextrin using the same procedure. The third group received structured dietary fiber advice, including portion size illustrations, to ensure appropriate dietary fiber intake for their age [16, 17]. All participants obtained the same conventional advice about diet, exercise, and behavior modification and monthly follow-up for 6 months.
Assessment of dietary intake, physical activity, anthropometry, body composition, and metabolic profiles
The details have been published elsewhere [15]. In brief, dietary intake was evaluated by a dietician using 3-day dietary records. The daily energy and nutrients intake were calculated using the Institute of Nutrition, Mahidol University Calculation-Nutrients (INMUCALs) Version 3 [18]. Physical activity was assessed by questionnaires. Anthropometry was collected by trained personnel and BMI Z-scores were calculated based on WHO 2007 growth reference using the WHO Anthroplus program [19]. Body composition was measured by bioelectrical impedance analysis using the InBody 770 (InBody Co., Ltd., Chungcheongnam-do, KOREA) and then fat mass index (FMI), and fat-free mass index (FFMI) were calculated [20]. For metabolic profiles, venous blood was attained after a 12-h fast to evaluate lipid profiles and alanine aminotransferase (ALT) which were determined according to the methods in the previous study [15].
Fecal collection, DNA isolation, and 16S PCR amplification
Fresh fecal samples were collected at baseline, the 3rd, and 6th month visits of the study for gut microbiota and SCFAs analyses. The details have been published elsewhere [5]. In brief, participants were provided with sterile stool collection kits, containers, and temperature-controlled packages and instructed to collect fecal samples at home. Each participant placed a fresh stool sample into one half of a 50 ml sterile container, which was then double-sealed in a zip-lock bag. The samples were stored in the freezer compartment of a home refrigerator (approximately −20 °C) and delivered to the laboratory within 24 h. Upon arrival, the samples were stored at −80 °C until analysis.
Fecal samples were resuspended in InhibitEX buffer (QIAGEN, Germany), incubated at 70 °C for 5 min, and centrifuged at 20,000 × g for 1 min. Supernatant was collected for DNA isolation by using QIAamp fast DNA stool mini kit (QIAGEN, Germany) in accordance with the manufacturer’s instructions and 16S PCR amplification was mentioned in the previous published study [5].
16S rRNA sequencing processing and analysis
Illumina paired-end reads of 16S rRNA from participants collected from the baseline, 3rd, and 6th month visits were analyzed as previously described [5]. Briefly, the quality of the data were inspected using FastQC [21]. All reads were processed by QIIME2 (v.2020.8) [22] and clustered at a similarity threshold of 97% to define operational taxonomic units (OTUs). Potential chimeric reads were removed using the q2-dada2 plugin [23] with default parameters. Taxonomic annotations were performed using the q2-feature-classifier plugin with the classify-sklearn option and confidence cutoff of 0.7. This method implements a Naïve Bayes classifier that was trained on the non-redundant SILVA 16s rRNA database (v.132) [24, 25]. Unclassified sequences were labeled as “unknown”.
Microbial functions and pathway abundances, based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) ontology, were annotated using the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States 2 (PICRUSt 2) software [26]. The abundance of each pathway was estimated based on the total copy number of genes involved in that pathway and the abundance of microbial taxa possessing those genes.
Differential analysis of relative taxonomic and KEGG pathway abundances
To normalize for different read depths across samples, abundance values were converted to percentages. Only genera and KEGG pathways that were detected (abundance >0) in at least 20% of the samples were retained. Overall, a total of 104 genera and 147 KEGG pathways were considered. The differences in abundance percentages between the 6th month and baseline were calculated for each participant and compared across the three groups using non-parametric tests (Mann–Whitney U test and Wilcoxon signed rank test). Alpha-diversity was measured using Shannon entropy and beta-diversity was calculated using Bray–Curtis dissimilarity.
Co-occurrence network analysis for KEGG pathways
To identify more important KEGG pathways beyond those whose abundances changed across treatment groups, we examined interactions between pathways through their co-occurrences. Pathways that significantly co-occurred with others (absolute Spearman’s correlation coefficient > 0.5 and adjusted p values < 0.05) were selected to construct a co-occurrence network. The betweenness centrality score was calculated using the networkx Python library and normalized to a scale of 0.0–1.0. Pathways with high normalized betweenness (> 0.5) were considered important in the context of inter-pathway interactions. For visualization, pathways whose abundances substantially changed between the 6th month and baseline (unadjusted Mann–Whitney U test p value < 0.05) were selected.
Short chain fatty acids
Fecal samples were prepared for analysis of SCFAs by diluting 10-fold with phosphate-buffered saline (pH 8.0) using a stomacher blender (Stomacher® 80 Biomaster; Seward, Worthing, UK) for 5 min as stated in the method of Kisuse et al. [27]. Then a 1 ml of fecal slurry was centrifuged at 13,000 × g for 5 min, and the supernatant was stored at −80 °C.
Lactic acid and SCFAs, including acetic acid, butyric acid, and propionic acid, from the fecal samples were investigated by high-performance liquid chromatography (Water 1525, USA). The samples were prepared by the method as previously defined with some modifications [28, 29]. All parameters were evaluated by Agilent Technologies 7890A equipment (Santa Clara, USA).
Statistical analysis
All statistical tests were performed using Python programming language (v.3.10.12). Differential abundance analyses with Mann–Whitney U tests and Wilcoxon signed rank tests were performed using the mannwhitneyu and the wilcoxon function from the scipy library. Spearman’s correlation coefficients were calculated using the spearmanr function from the scipy library, which also reported p values. Benjamini–Hochberg corrections were performed and an adjusted p value cutoff of 0.05 was applied to define statistical significance. Associations between changes in taxonomic or pathway abundances and changes in clinical features were measured using Pearson’s correlation coefficients.
Results
A total of 165 Thai children with obesity participated in the study (mean age: 10.4 ± 2.2 years, 59% male). They were randomly allocated to the placebo, inulin, and dietary fiber advice groups. Only 143 participants who completed the study with available taxonomic and functional pathway abundance profiles at the baseline, 3rd, and 6th month visits were included in this study. There was no difference in the attrition rate between the three groups. Neither participants receiving inulin nor those receiving placebo had any significant side effects [15].
Baseline characteristics and gut microbiota
Demographic data and baseline characteristics of all groups were illustrated elsewhere [15]. There were no significant differences in baseline anthropometry, clinical data, nutrient intake, physical activity, or biochemical markers. Moreover, baseline gut microbiota diversity and composition were not significantly different among the three groups (p > 0.05) (Table 1).
Effects on gut microbiota diversity and composition
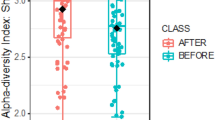
Based on community-wide analysis, there were 15 phyla, 118 families, and 340 genera detected in our study. There was no significant difference in beta-diversity among the three groups at the phylum level and the genus level (Supplementary Fig. 1). Alpha-diversity (Shannon Index), the index assessing the variety of intestinal microbiota, was not significantly different at the phylum level. However, the alpha-diversity greatly increased in the inulin group compared to the placebo and dietary fiber advice groups at the genus level (p = 0.028 and p = 0.026) (Fig. 1). In addition, the alpha-diversity significantly increased from baseline to the 6th month in the inulin group (p = 0.033). The other groups exhibited no significant change.
The changes of microbial abundance at the phylum level from month 0 and month 6 were shown in Supplementary Fig. 2. There were no significant differences in the change of relative abundance of Actinobacteria (Supplementary Fig. 2A), Bacteroidetes (Supplementary Fig. 2B), Firmicutes (Supplementary Fig. 2C), Fusobacteria, and Proteobacteria (data not shown) among the placebo, inulin, and dietary fiber advice groups.
However, at the genus level, a substantial elevation in the relative abundance of Bifidobacterium was found after 6-month inulin supplementation compared to the placebo and dietary fiber advice groups (p = 0.0058 and p = 0.025, respectively) (Fig. 2A). Notably, the results showed a significant enrichment of butyrate-producing bacteria, Agathobacter, in the inulin group compared to the placebo group (p = 0.04), and this genus had a tendency of elevation compared to the dietary fiber advice group (p = 0.05) (Fig. 2B). Inulin supplementation greatly enhanced Eubacterium coprostanoligenes compared to the placebo group (p = 0.04) (Fig. 2C). A significant increase in the relative abundance of the other bacteria in the group of butyrate-producing bacteria, Subdoligranulum, was found after the 6-month inulin supplementation compared to the placebo group (p = 0.009) (Fig. 2D). The dietary fiber advice group also showed an increase in Subdoligranulum compared to the placebo group (p = 0.04). Blautia was greatly enhanced after inulin supplementation compared to the dietary fiber advice group (p = 0.03). Megasphaera was significantly increased in the inulin group compared to the placebo group (p = 0.03) and had a tendency of elevation compared to the dietary fiber advice group (p = 0.088).
For within group analysis, Bifidobacterium was significantly increased from baseline to the 6th month in the inulin group (p = 0.0025), but this result was not observed in the placebo and dietary fiber advice groups. Within-group analysis showed significant differences in the relative abundance of Agathobacter, Eubacterium coprostanoligenes, and Subdoligranulum in the inulin group (p = 0.016, p = 0.04, and p = 0.049, respectively). While there was no significant difference in relative abundance of these SCFA-producing bacteria from baseline to the 6th month in the other groups. Blautia was significantly increased in the inulin group after the 6-month study period, whereas no significant difference of this genus was found in the other groups. Moreover, the levels of Megasphaera tended to increase from baseline to the 6th visit only in the inulin group (p = 0.08). The microbial metabolites, fecal SCFAs, tended to increase in all groups, whereas no significant difference between groups was observed (data not shown).
Changes in microbial functions
The change of microbial functions between groups after the 6-month study period was assessed. The proteasome pathway (ko03050) was greatly upregulated in the inulin group compared to the placebo and dietary fiber advice groups (p = 0.017 and p = 0.028, respectively) (Fig. 3A). The riboflavin metabolism pathway (ko00740) significantly increased in the placebo group compared to the inulin group (p = 0.027) (Fig. 3B). Within group analysis of the changes in pathway abundances, demonstrated as co-occurrence networks (Supplementary Fig. 3A–C), showed that different pathways were impacted among the three groups. For instance, the proteosome pathway (ko03050) and the ribosome biogenesis pathway (ko03008) were substantially increased only in the inulin group while the histidine metabolism pathway (ko00340) was significantly elevated in the placebo and dietary fiber advice groups.
The relationship between the changes in gut microbiota and the changes in clinical and metabolic features after the 6-month period was notable in the inulin group (Fig. 4B) compared to the placebo and dietary fiber advice groups (Fig. 4A, C). Regarding the inulin group, Faecalibacterium abundance change positively correlated with the change of butyrate. The change in Fusobacterium abundance was negatively associated with ALT. Parasutterella abundance change was positively correlated with the change in adiposity, including FM, FMI, trunk FM, trunk FMI, and visceral fat area (VFA). The change in Romboutsia abundance was negatively correlated with FFM and FFMI, but positively correlated with VFA. The change in Eubacterium coprostanoligenes group was negatively correlated with high energy intake. The change in Subdoligranulum abundance was negatively associated with BMI Z-score. However, these relationship patterns were completely unobserved in the placebo and dietary fiber advice groups.
A Placebo group, B Inulin group, and C Dietary fiber advice group. ALT alanine aminotransferase, Chol_intake cholesterol intake, DF dietary fiber intake, DFperC dietary fiber intake, g per 1000 kcal, Fat_distri fat distribution, FFM fat-free mass, FFMI fat-free mass index, FM fat mass, FMI fat mass index, H_dwk high intensity exercise, days per week, H_minwk high intensity exercise, minutes per week, Screen day screen time on weekday, Screen end screen time on weekend, TC total calories, TG triglyceride, VFA visceral fat area.
Discussion
As far as we know, this is the largest randomized control trial (RCT) study with gut microbiota and metabolomic evaluations of a prebiotic intervention in children with obesity. Here, inulin enhanced several SCFA-producing bacteria, especially Bifidobacterium spp., and increased proteasome pathway with decreased riboflavin metabolism (microbial function analysis) when compared to the control. As such, obesity induced gut dysbiosis [30, 31] that was attenuated by inulin as indicated by the increased variety of gut microbiota (alpha-diversity) after the 6-month intervention. Although a previous RCT using OI did not demonstrate alterations in alpha-diversity, the elevation of Bifidobacterium spp. [12] was mentioned similar to the use of other prebiotics [10, 11]. Additionally, several inulin-induced bacteria as we found were SCFA-producing bacteria with the well-known benefits against obesity. Accordingly, Agathobacter was found in adults with obesity achieving weight control after 12-week high fiber rye administration [32] and in patients receiving oatmeal [33]. Eubacterium coprostanoligenes was found in the healthy individuals [34] with an essential role of SCFA production [35], and Subdoligranulum regulates host energy and reduced inflammation [36] with negatively associated with adipocyte and other metabolic disturbances [37]. Blautia was found primarily in the lean individuals [38] and the well-controlled type 2 diabetes cases [39], while Megasphaera was elevated in the individuals with obesity after treatment [40]. Based on clinical results from our previously published study, we found a significant increase in fat-free mass along with enhancement of Bifidobacterium and several SCFA-producing bacteria only in the inulin group. Optimizing the intestinal microbial ecosystem has been linked to reduced inflammation and increased anti-inflammatory activity [12]. Bifidobacterium metabolizes inulin to produce substrates necessary for other SCFA-producing bacteria, thus facilitating SCFA synthesis [41]. Taken together, metabolites derived from these bacteria would manifest anti-inflammatory activity, then possibly promoting myogenesis [42] as we found fat-free mass gain. Further study of gut-muscle axis response from inulin supplementation in childhood obesity is warranted. Despite the possible influences of SCFAs in obesity, our results demonstrated a similar pattern of fecal SCFA profiles among the three groups which might be because these metabolites were quickly absorbed into the body, making it difficult to detect the difference in feces.
Interestingly, the anti-inflammatory SCFAs might attenuate obesity-induced chronic inflammation that is partly modulated through inflammasome pathway [43, 44], especially nuclear factor-κB (NF-κB) and activator protein 1 [45, 46]. Because proteasomes partly regulate NF-κB pathway [47], modulation of proteasome-dependent NF-κB; for example, through inulin supplement, might conserve gut homeostasis. Conversely, riboflavin regulates the pro-inflammatory activity of macrophages and plays a key role in mediating obesity-related inflammation [48, 49]. Its metabolism significantly increased in the placebo group compared to the inulin group, indicating that inulin might attenuate inflammation resulting in less riboflavin metabolism for inflammatory process.
Additionally, within group analysis of functional changes in the inulin group showed a different pattern of significant pathways compared to the other groups. Proteasome pathway significantly increased only in the inulin group, consistent with between-group analysis. Stazar et al. found that histidine metabolism positively affects tumor necrosis factor-α stimulation in African participants [50]. In our study, this pathway significantly increased in the placebo and dietary fiber advice groups, but remained unchanged in the inulin group, suggesting that inulin might mitigate inflammation. The ribosome biogenesis plays an essential role in protein synthesis, leading to regulation of skeletal muscle growth and muscle building. This finding agreed with the result of fat-free mass gain in our previous RCT study [15].
Notably, we found several relationships between the changes in gut microbiota and the changes in clinical and metabolic features exclusively in the inulin group. For example, high relative abundance of Parasutterella involved in systemic low-grade inflammation and activation of the fatty acid biosynthesis pathway leading to weight gain [51], as we found this genus had a positive direction to adiposity. We observed a positive relationship between Romboutsia and adiposity, consistent with previous studies linking Romboutsia to obesity [52]. Eubacterium coprostanoligenes group revealed a negative relationship with body weight and cholesterol in animal models [53] and a longitudinal study demonstrated that Subdoligranulum was negatively associated with future BMI in childhood [54]. The findings of both genera were relevant to our results. All these relationship patterns in the inulin group suggested that inulin supplementation might establish favorable connections of gut microbiota with clinical features and biochemical markers.
Strengths and limitations of the study
This present study appears to be the largest RCT documenting the change of gut microbiota and microbial functions after prebiotic supplementation in children with obesity. The study indicated that the supplementation of the extracted inulin from Thai Jerusalem artichoke showed an effect on enrichment of favorable microbes with a novel finding of an increased alpha-diversity in children with obesity. We used next-generation sequencing technology, the 16S rRNA sequencing, to analyze the bacterial sequences, which was a ubiquitous technique to identify bacterial community in the intestine. The limitation of this study was the assessment of fecal SCFAs which might be difficult to detect any difference between groups. However, the results of fecal SCFAs might not explain an entire process of metabolic changes. Further exploration of serum SCFAs could lead to better understanding of host energy harvest and storage mechanism.
Conclusions
Inulin supplementation could be a strategic treatment to selectively promote beneficial microbes and restore the balance of intestinal microbiota in children with obesity. Moreover, the predominantly functional characteristics of the gut microbiota and their associations with clinical and metabolic parameters could be attributed to inulin supplementation, leading to different patterns from the placebo and dietary fiber advice groups. Further exploration of the impacts of inulin supplementation as a long-term intervention on gut microbiota and their metabolites could elicit deeper knowledge of host-microbe interaction in pediatric obesity.
Data availability
Data described in the manuscript will be made available upon request pending application and approval from the corresponding author. 16S rRNA sequencing data (FASTQ files) were submitted to the Sequence Read Archive (SRA) under the reference PRJNA1096061.
References
World Health Organization. Obesity and overweight. 2018. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
Sahoo K, Sahoo B, Choudhury AK, Sofi NY, Kumar R, Bhadoria AS. Childhood obesity: causes and consequences. J Fam Med Prim Care. 2015;4:187–92.
Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81.
Gérard P. Gut microbiota and obesity. Cell Mol Life Sci. 2016;73:147–62.
Visuthranukul C, Sriswasdi S, Tepaamorndech S, Joyjinda Y, Saengpanit P, Kwanbunbumpen T, et al. Association of human intestinal microbiota with lifestyle activity, adiposity, and metabolic profiles in Thai children with obesity. J Nutr Metab. 2022;2022:3029582.
Nirmalkar K, Murugesan S, Pizano-Zárate ML, Villalobos-Flores LE, García-González C, Morales-Hernández RM, et al. Gut microbiota and endothelial dysfunction markers in obese Mexican children and adolescents. Nutrients. 2018;10:2009.
Shin S, Cho KY. Altered gut microbiota and shift in bacteroidetes between young obese and normal-weight Korean children: a cross-sectional observational study. Biomed Res Int. 2020;2020:6587136.
Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol. 2015;12:303–10.
Meyers GR, Samouda H, Bohn T. Short chain fatty acid metabolism in relation to gut microbiota and genetic variability. Nutrients. 2022;14:5361.
Salazar N, Dewulf EM, Neyrinck AM, Bindels LB, Cani PD, Mahillon J, et al. Inulin-type fructans modulate intestinal Bifidobacterium species populations and decrease fecal short-chain fatty acids in obese women. Clin Nutr. 2015;34:501–7.
Dewulf EM, Cani PD, Claus SP, Fuentes S, Puylaert PG, Neyrinck AM, et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut. 2013;62:1112–21.
Nicolucci AC, Hume MP, Martinez I, Mayengbam S, Walter J, Reimer RA. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology. 2017;153:711–22.
Hume MP, Nicolucci AC, Reimer RA. Prebiotic supplementation improves appetite control in children with overweight and obesity: a randomized controlled trial. Am J Clin Nutr. 2017;105:790–9.
Liber A, Szajewska H. Effect of oligofructose supplementation on body weight in overweight and obese children: a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2014;112:2068–74.
Visuthranukul C, Chamni S, Kwanbunbumpen T, Saengpanit P, Chongpison Y, Tepaamorndech S, et al. Effects of inulin supplementation on body composition and metabolic outcomes in children with obesity. Sci Rep. 2022;12:13014.
Keim NL, Levin RJ, Havel PJ. Dietary fiber. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, editors. Modern nutrition in health and disease. Philadelphia: Lippincott Williams & Wilkins; 2012. pp. 58–64.
Bureau of Nutrition, Department of Health, Ministry of Public Health. Thai food composition table. 2010. http://nutrition.anamai.moph.go.th/images/file/fruit_nutritioin3.pdf.
Banjong O, Wanijjakul C, Peemanee K, Ananthasuk S. Application manual: INMUCAL-Nutrients V.3 Institute of Nutrition, Mahidol University. 13th ed. Nakhon Pathom: Metta Copy Print Limited Partnership; 2016.
Blossner M, Siyam A, Borghi E, Onyango A, de Onis M. WHO AnthroPlus for personal computers manual: software for assessing growth of the world’s children and adolescents. Geneva, Switzerland: Department of Nutrition for Health and Development, World Health Organization; 2009. https://www.who.int/growthref/tools/en/.
Wells JC, Cole TJ. Adjustment of fat-free mass and fat mass for height in children aged 8 y. Int J Obes Relat Metab Disord. 2002;26:947–52.
Babraham IB. Bioinformatics. 2021. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37:852–7.
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–3.
Bokulich NA, Kaehler BD, Rideout JR, Dillon M, Bolyen E, Knight R, et al. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome. 2018;6:90.
Robeson MS 2nd, O’Rourke DR, Kaehler BD, Ziemski M, Dillon MR, Foster JT, et al. RESCRIPt: reproducible sequence taxonomy reference database management. PLoS Comput Biol. 2021;17:e1009581.
Douglas GM, Maffei VJ, Zaneveld JR, Yurgel SN, Brown JR, Taylor CM, et al. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol. 2020;38:685–8.
Kisuse J, La-Ongkham O, Nakphaichit M, Therdtatha P, Momoda R, Tanaka M, et al. Urban diets linked to gut microbiome and metabolome alterations in children: a comparative cross-sectional study in Thailand. Front Microbiol. 2018;9:1345.
Wang HY, Wang C, Guo LX, Zheng YF, Hu WH, Dong TTX, et al. Simultaneous determination of short-chain fatty acids in human feces by HPLC with ultraviolet detection following chemical derivatization and solid-phase extraction segmental elution. J Sep Sci. 2019;42:2500–9.
Sathitkowitchai W, Suratannon N, Keawsompong S, Weerapakorn W, Patumcharoenpol P, Nitisinprasert S, et al. A randomized trial to evaluate the impact of copra meal hydrolysate on gastrointestinal symptoms and gut microbiome. PeerJ. 2021;9:e12158.
Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–6.
Graessler J, Qin Y, Zhong H, Zhang J, Licinio J, Wong ML, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013;13:514–22.
Iversen KN, Dicksved J, Zoki C, Fristedt R, Pelve EA, Langton M, et al. The effects of high fiber rye, compared to refined wheat, on gut microbiota composition, plasma short chain fatty acids, and implications for weight loss and metabolic risk factors (the RyeWeight Study). Nutrients. 2022;14:1669.
Xu D, Pan D, Liu H, Yang C, Yang X, Wang X, et al. Improvement in cardiometabolic risk markers following an oatmeal diet is associated with gut microbiota in mildly hypercholesterolemic individuals. Food Res Int. 2022;160:111701.
Hu J, Guo P, Mao R, Ren Z, Wen J, Yang Q, et al. Gut microbiota signature of obese adults across different classifications. Diabetes Metab Syndr Obes. 2022;15:3933–47.
Mukherjee A, Lordan C, Ross RP, Cotter PD. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut microbes. 2020;12:1802866.
Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277.
Van Hul M, Le Roy T, Prifti E, Dao MC, Paquot A, Zucker JD, et al. From correlation to causality: the case of Subdoligranulum. Gut microbes. 2020;12:1–13.
Bombin A, Yan S, Bombin S, Mosley JD, Ferguson JF. Obesity influences composition of salivary and fecal microbiota and impacts the interactions between bacterial taxa. Physiol Rep. 2022;10:e15254.
Tong X, Xu J, Lian F, Yu X, Zhao Y, Xu L, et al. Structural alteration of gut microbiota during the amelioration of human type 2 diabetes with hyperlipidemia by metformin and a traditional chinese herbal formula: a multicenter, randomized, open label clinical trial. mBio. 2018;9:e02392–17.
Kang Y, Li Y, Du Y, Guo L, Chen M, Huang X, et al. Konjaku flour reduces obesity in mice by modulating the composition of the gut microbiota. Int J Obes. 2019;43:1631–43.
Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294:1–8.
Przewłócka K, Folwarski M, Kaźmierczak-Siedlecka K, Skonieczna-Żydecka K, Kaczor JJ. Gut-muscle axis exists and may affect skeletal muscle adaptation to training. Nutrients. 2020;12:1451.
Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8:42.
Saad MJ, Santos A, Prada PO. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology. 2016;31:283–93.
Mohapatra G, Eisenberg-Lerner A, Merbl Y. Gatekeepers of the gut: the roles of proteasomes at the gastrointestinal barrier. Biomolecules. 2021;11:989.
Inoue S, Nakase H, Matsuura M, Mikami S, Ueno S, Uza N, et al. The effect of proteasome inhibitor MG132 on experimental inflammatory bowel disease. Clin Exp Immunol. 2009;156:172–82.
Kravtsova-Ivantsiv Y, Ciechanover A. The ubiquitin-proteasome system and activation of NF-κB: involvement of the ubiquitin ligase KPC1 in p105 processing and tumor suppression. Mol Cell Oncol. 2015;2:e1054552.
Mazur-Bialy AI, Pocheć E. HMGB1 inhibition during zymosan-induced inflammation: the potential therapeutic action of riboflavin. Arch Immunol Ther Exp. 2016;64:171–6.
Mazur-Bialy AI, Pocheć E. Riboflavin reduces pro-inflammatory activation of adipocyte-macrophage co-culture. Potential application of vitamin B2 enrichment for attenuation of insulin resistance and metabolic syndrome development. Molecules. 2016;21:1724.
Stražar M, Temba GS, Vlamakis H, Kullaya VI, Lyamuya F, Mmbaga BT, et al. Gut microbiome-mediated metabolism effects on immunity in rural and urban African populations. Nat Commun. 2021;12:4845.
Xiao S, Liu C, Chen M, Zou J, Zhang Z, Cui X, et al. Scutellariae radix and coptidis rhizoma ameliorate glycolipid metabolism of type 2 diabetic rats by modulating gut microbiota and its metabolites. Appl Microbiol Biotechnol. 2020;104:303–17.
Wu C, Lyu W, Hong Q, Zhang X, Yang H, Xiao Y. Gut microbiota influence lipid metabolism of skeletal muscle in pigs. Front Nutr. 2021;8:675445.
Chen YT, Hsu AH, Chiou SY, Lin YC, Lin JS. AB-Kefir reduced body weight and ameliorated inflammation in adipose tissue of obese mice fed a high-fat diet, but not a high-sucrose diet. Nutrients. 2021;13:2182.
Houtman TA, Eckermann HA, Smidt H, de Weerth C. Gut microbiota and BMI throughout childhood: the role of firmicutes, bacteroidetes, and short-chain fatty acid producers. Sci Rep. 2022;12:3140.
Acknowledgements
The study was conducted by the Center of Excellence in Pediatric Nutrition, Division of Nutrition, Department of Pediatrics, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. The authors thank Professor Thiravat Hemachudha, MD for supporting us in the next-generation sequencing process; Professor Chitsanu Pancharoen, MD for supporting us with enrollment; Professor Vorasuk Shotelersuk, MD and Professor Dr. Kanya Supapeetiporn, MD, PhD for assistance with funding; Dr. Massalin Nakphaichit, PhD, Jaraspong Uaariyapanichkul, MD, Puthita Saengpanit, MD, and Ekkarit Panichsillaphakit, MD for assistance with study information; and Chula Clinical Research Center (Chula CRC), Faculty of Medicine, Chulalongkorn University for research assistant training. The authors appreciate the effort and dedication of all the researchers, data collection team, and everyone involved in this project. The authors sincerely thank all the children and their parents for their participation in this study.
Funding
This research was funded by the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University [Grant no. RA60/122]; the Center of Excellence in Pediatric Nutrition, Faculty of Medicine, Chulalongkorn University; and received external funding by NSTDA Research Fund [Grant no. FDA-CO-2561-5614-TH], Ministry of Higher Education, Science, Research and Innovation, Thailand.
Author information
Authors and Affiliations
Contributions
Authorships are based on fulfillment of the criteria recommended by the International Committee of Medical Journal Editors (ICMJE). CV was the principal investigator, responsible for the design and conduct of the study, involved in the monitoring and acquisition of clinical data, laboratory analysis, and interpretation of data, performed the statistical analysis, and wrote the research article. SS was involved in the monitoring and acquisition of the laboratory analysis, interpretation of data, performed the statistical analysis, made the figures, and jointly wrote the research article. ST was involved in the monitoring and acquisition of the laboratory analysis, interpretation of data, and jointly wrote the research article. SC (Supakarn Chamni) was involved in the monitoring and acquisition of the laboratory analysis, interpretation of data, and jointly wrote the research article. AL was involved in the monitoring and acquisition of the laboratory analysis, interpretation of data, and jointly wrote the research article. YJ was involved in the monitoring and acquisition of the laboratory analysis and interpretation of data. VA was involved in the monitoring and acquisition of the laboratory analysis and interpretation of data. SC (Sirinuch Chomtho) was involved in the design and conduct of the study, monitoring and acquisition of clinical data and laboratory analysis, interpretation of data, and jointly wrote the research article. All authors critically reviewed the manuscript, and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The research involving human subjects, human material, and human data was in accordance with the Declaration of Helsinki. The study was approved by the Institutional Review Board, Faculty of Medicine, Chulalongkorn University (IRB no. 240/60). Informed assent and consent were obtained from all participants and their parents prior to enrollment.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Visuthranukul, C., Sriswasdi, S., Tepaamorndech, S. et al. Enhancing gut microbiota and microbial function with inulin supplementation in children with obesity. Int J Obes (2024). https://doi.org/10.1038/s41366-024-01590-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41366-024-01590-8
- Springer Nature Limited