Abstract
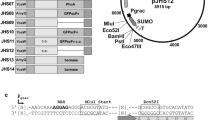
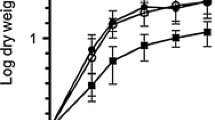
Using the gene for a secreted protease inhibitor, Streptomyces subtilisin inhibitor (SSI), we have established a secretory expression system for the products of foreign genes in Streptomyces. A sex pheromone peptide, cADl, of Enterococcus faecalis was expressed and secreted as a stable complete fusion protein with SSI in amounts comparable to the native SSI in S. lividans 66. Recombinant cADl, isolated from the fusion protein by chemical digestion with BrCN, has the same amino acid composition and sequence, retention time on reverse phase HPLC, molecular mass, and biological activity as authentic cADl. This system should be useful for the efficient expression of other foreign gene products, and as a model for heterologous gene expression in Streptomyces.
Similar content being viewed by others
References
Gray, G., Selzer, G., Buell, G., Shaw, P., Escanez, S., Hofer, S., Voegeli, P., and Thompson, C.J. 1984. Synthesis of bovine growth hormone by Streptomyces lividans. Gene 32:21–30.
Munoz, A., Perez-Aranda, A., and Barbero, J.L. 1985. Cloning and expression of human interleukin 2 in Streptomyces lividans using the Escherichia coli consensus promoter. Biochem. Biophys. Res. Comm. 133:511–519.
Ali, N.A., and Dale, J.W. 1986. Secretion by Streptomyces lividans of a cloned Gram-negative beta-lactamase. FEMS Microbiol. Lett. 33:277–280.
Pulido, Diego., Vara, J.A. and Jimenez, A. 1986. Cloning and expression in biologically active form of the gene for human interferon α2 in Streptomyces. Gene 45:167–174.
Noack, D., Geuther, R., Tonew, M., Breitling, R. and Behnke, D. 1988. Expression and secretion of interferon-α1 by Streptomyces lividans: use of staphylokinase signals and amplification of a neo gene. Gene 68:53–62.
Obata, S., Taguchi, S., Kumagai, I., and Miura, K. 1989. Molecular cloning and nucleotide sequence determination of the gene encoding Streptomyces Subtilisin Inhibitor (SSI). J. Biochem. 105:367–371.
Obata, S., Furukubo, S., Kumagai, I., Takahashi, H. and Miura, K. 1989. High-level expression in Streptomyces Iividans 66 of a gene encoding Streptomyces Subtilisin Inhibitor from Streptomyces albognseolus S-3253. J. Biochem. 105:372–376.
Mori, M., Sakagami, Y., Narita, M., Isogai, A., Fujino, M., Kitada, C., Craig, R.A., Clewell, D.B., and Suzuki, A. 1985. Isolation and structure of the bacterial sex pheromone, cAD1, that induces plasmid transfer in Streptococcus faecalis. FEBS Lett. 178:97–100.
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685.
Ouchterlony, O. 1967. Handbook of Experimental Immunology, Weir, D. M. (Ed.). Blackwell Scientific, Oxford, Edinburgh, p. 676.
Murao, S., Sato, S., and Muto, N. 1972. Isolation of alkaline protease inhibitor from Streptomyces albogiseolus S-3253. Agr. Biol. Chem. 36:1737–1744.
Hiromi, K., Akasaka, K., Mitsui, Y., Tonomura, B. and Murao, S. ed. 1985. Protein Protease Inhibitor-The case of Streptomyces Subtilisin (SSI) Inhibitor Chapters 7, 9 (SSI). Elsevier Science Publishers B. V., Amsterdam.
Crestfield, A.M., Moore, S. and Stein, W.H. 1963. The preparation and enzymatic hydolysis of reduced and S-carboxymethylated proteins. J. Biol. Chem. 238:622–627.
Gross, E., and Witkop, B. 1961. Selective cleavage of the methionyl peptide bonds in ribonuclease with cyanogen bromide. J. Amer. Chem. Soc 83:1510–1511.
Dunny, G.M., Craig, R.L., Carron, R.B., and Clewell, D.B. 1979. Plasmid transfer in Streptococcus faecalis: Production of multiple sex pheromone by recipients. Plasmid 2:454–465.
Nagai, K., Thogerson, H.C. 1984. Generation of β-globin by sequence-specific proteolysis of a hybrid protein produced in Escherichia coli. Nature 309:810–812.
Hattori, M., and Sakaki, Y. 1986. Dideoxy sequencing using denatured plasmid templates. Anal. Biochem. 152:232–238.
Morinaga, Y., Franceschini, T., Inouye, S., Inouye, M. 1984. Improvement of oligonucleotide-directed site-specific mutagenesis using double-stranded plasmid DNA. BioTechnology 2:636–639.
Hunter, I.S. 1985. DNA Cloning: A Practical Approach, Vol. 2. Glover, D. M. (Ed.) IRL Press.
Bensadoun, A. and Weinstein, D. 1976. Assay of proteins in the presence of interfering materials. Anal. Biochem. 70:241–250.
L'Italien, J.J. and Strickler, J.E. 1982. Application of high-performance liquid chromatographic peptide purification to protein microsequencing by solid-phase Edman degradation. Anal. Biochemistry 127:198–212.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Taguchi, S., Kumagai, I., Nakayama, J. et al. Efficient Extracellular Expression of a Foreign Protein in Streptomyces Using Secretory Protease Inhibitor (SSI) Gene Fusions. Nat Biotechnol 7, 1063–1066 (1989). https://doi.org/10.1038/nbt1089-1063
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt1089-1063
- Springer Nature America, Inc.