Abstract
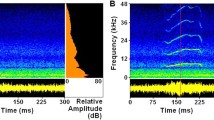
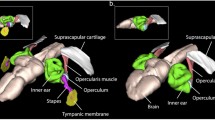
Sound communication plays a vital role in frog reproduction1,2, in which vocal advertisement is generally the domain of males. Females are typically silent, but in a few anuran species they can produce a feeble reciprocal call3 or rapping sounds4 during courtship. Males of concave-eared torrent frogs (Odorrana tormota) have demonstrated ultrasonic communication capacity5. Although females of O. tormota have an unusually well-developed vocal production system6, it is unclear whether or not they produce calls or are only passive partners in a communication system dominated by males. Here we show that before ovulation, gravid females of O. tormota emit calls that are distinct from males’ advertisement calls, having higher fundamental frequencies and harmonics and shorter call duration. In the field and in a quiet, darkened indoor arena, these female calls evoke vocalizations and extraordinarily precise positive phonotaxis (a localization error of <1°), rivalling that of vertebrates with the highest localization acuity (barn owls7,8, dolphins, elephants and humans9). The localization accuracy of O. tormota is remarkable in light of their small head size (interaural distance of <1 cm), and suggests an additional selective advantage of high-frequency hearing beyond the ability to avoid masking by low-frequency background noise5.


Similar content being viewed by others
References
Capranica, R. R. The Evoked Vocal Response of the Bullfrog: A Study of Communication by Sound (Research Monograph 33, MIT, Cambridge, Massachusetts, 1965)
Glaw, F. & Vences, M. A Fieldguide to the Amphibians and Reptiles of Madagascar 2nd edn (Vences & Glaw, Köln, Germany, 1994)
Roy, D. Communication signals and sexual selection in amphibians. Curr. Sci. 72, 923–927 (1997)
Tobias, M. L., Viswanathan, S. & Kelley, D. B. Rapping, a female receptive call, initiates male/female duets in the South African clawed frog. Proc. Natl Acad. Sci. USA 95, 1870–1875 (1998)
Feng, A. S. et al. Ultrasonic communication in frogs. Nature 440, 333–336 (2006)
Suthers, R. A. et al. Voices of the dead: Complex nonlinear vocal signals from the larynx of an ultrasonic frog. J. Exp. Biol. 209, 4984–4993 (2006)
Knudsen, E. I., Blasdel, G. G. & Konishi, M. Sound localization by the barn owl (Tyto alba) measured with search coil technique. J. Comp. Physiol. A 133, 1–11 (1979)
Bala, A. D. S., Spitzer, M. W. & Takahashi, T. T. Prediction of auditory spatial acuity from neural images on the owl’s auditory space map. Nature 424, 771–774 (2003)
Heffner, R. S. & Masterton, R. B. in Comparative Perception Vol. I, Basic Mechanisms (eds Berkley, M. A. & Stebbins, W. C.) 285–314 (John Wiley & Sons, New York, 1990)
Cai, H.-X., Che, J., Pang, J.-F., Zhao, E.-M. & Zhang, Y.-P. Paraphyly of Chinese Amolops (Anura, Ranidae) and phylogenetic position of the rare Chinese frog, Amolops tormotus . Zootaxa 1531, 49–55 (2007)
Narins, P. M. et al. Old world frog and bird vocalizations contain prominent ultrasonic harmonics. J. Acoust. Soc. Am. 115, 910–913 (2004)
Feng, A. S., Narins, P. M. & Xu, C. H. Vocal acrobatics in a Chinese frog, Amolops tormotus . Naturwissenschaften 89, 352–356 (2002)
Narins, P. M. & Smith, S. L. Clinal variation in anuran advertisement calls: Basis for acoustic isolation? Behav. Ecol. Sociobiol. 19, 135–141 (1986)
Marquez, R. Female choice in the midwife toads (Alytes obstetricans and A. cisternasii). Behaviour 132, 151–161 (1995)
McClelland, B. E., Wilczynski, W. & Ryan, M. J. Correlations between call characteristics and morphology in male cricket frogs (Acris crepitans). J. Exp. Biol. 199, 1907–1919 (1996)
Gerhardt, H. C. & Huber, F. Acoustic Communication in Insects and Anurans (Univ. Chicago Press, Chicago, 2002)
Narins, P. M., Feng, A. S., Fay, R. R. & Popper, A. N. Hearing and Sound Communication in Amphibians (Springer, New York, 2007)
Duellman, W. E. & Trueb, L. Biology of Amphibians (McGraw-Hill, New York, 1986)
Roy, D., Borah, B. & Sarma, A. Analysis and significance of female reciprocal call in frogs. Curr. Sci. 69, 265–270 (1995)
Kelley, D. B. & Tobias, M. L. in The Design of Animal Communication (eds Konishi, M. & Hauser, M.) 9–35 (MIT Press, Cambridge, Massachusetts, 1999)
Emerson, S. B. & Boyd, S. K. Mating vocalizations of female frogs: Control and evolutionary mechanisms. Brain Behav. Evol. 53, 187–197 (1999)
Kelley, D. B. Vocal communication in frogs. Curr. Opin. Neurobiol. 14, 751–757 (2004)
Rand, A. S. Is the canary singing? Froglog 48, 3 (2001)
Christensen-Dalsgaard, J. in Sound Source Localization (eds Popper, A. N. & Fay, R. R.) 67–123 (Springer, New York, 2005)
Siemers, B. M. & Schnitzler, H.-U. Echolocation signals reflect niche differentiation in five sympatric congeneric bat species. Nature 429, 657–661 (2004)
Acknowledgements
This work was supported by the National Natural Sciences Foundation of China (J.-X.S.), the National Institute on Deafness and Other Communication Disorders (A.S.F. and P.M.N.), the UCLA Academic Senate and the Paul S. Veneklasen Research Foundation (P.M.N.), and the National Science Foundation (A.S.F.).
Author Contributions J.-X.S., A.S.F. and P.M.N. were responsible for project planning. All authors (except P.M.N.) conducted the behavioural experiments, J.-X.S. and Z.-M.X. analysed the behavioural data and video recordings, and J.-X.S. and A.S.F. analysed the acoustic data. All authors contributed to writing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Audio 1
The file contains Supplementary Audio 1 with phonotactic behaviour of males of Odorrana tormota in response to female courtship calls. In response to female courtship calls (FCs), males of O. tormota produced four types of vocal responses and displayed phonotactic movement toward the loudspeaker. (WMV 1552 kb)
Supplementary Audio 2
The file contains Supplementary Audio 2 with males of Odorrana tormota show hyperacute phonotaxis to female courtship calls. Upon hearing a female courtship call, a male of O. tormota usually oriented his body toward the loudspeaker – this was followed by a long-distance hop (range: 30 - 75 cm) toward the loudspeaker. The precision of the long-distance hops was remarkable, and the male soon reached the center of the diaphragm of the loudspeaker. (WMV 1208 kb)
Rights and permissions
About this article
Cite this article
Shen, JX., Feng, A., Xu, ZM. et al. Ultrasonic frogs show hyperacute phonotaxis to female courtship calls. Nature 453, 914–916 (2008). https://doi.org/10.1038/nature06719
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06719
- Springer Nature Limited
This article is cited by
-
Frequency jumps and subharmonic components in calls of female Odorrana tormota differentially affect the vocal behaviors of male frogs
Frontiers in Zoology (2023)
-
Albert Feng: father, friend, scientist, innovator (1944–2021)
Journal of Comparative Physiology A (2023)
-
Behind the mask(ing): how frogs cope with noise
Journal of Comparative Physiology A (2023)
-
DPOAEs and tympanal membrane vibrations reveal adaptations of the sexually dimorphic ear of the concave-eared torrent frog, Odorrana tormota
Journal of Comparative Physiology A (2023)
-
Neuroethology of sound localization in anurans
Journal of Comparative Physiology A (2023)