Abstract
Background:
Recent studies have demonstrated that microRNAs are stably detectable in plasma/serum because of their binding to specific proteins or being packaged in secretory particles. This study was designed to detect novel microRNAs in plasma for cancer detection and monitoring using microRNA array-based approaches in oesophageal squamous cell carcinoma (ESCC) patients.
Methods:
Through the integration of two Toray 3D-Gene microRNA array-based approaches to compare plasma microRNA levels between ESCC patients and healthy volunteers and between preoperative and postoperative ESCC patients, we identified a novel plasma biomarker in ESCC.
Results:
(1) Eight upregulated and common microRNAs (miR-15b, 16, 17, 25, 19b, 20a, 20b, and 106a) were selected using two high-resolution microRNA array approaches. (2) Test-scale analyses by quantitative RT–PCR validated a significant higher levels of plasma miR-19b (P=0.0020) and miR-25 (P=0.0030) in ESCC patients than controls. However, a significant correlation was observed between plasma miR-19b levels and concentrations of red blood cells (P=0.0073) and haemoglobin (P=0.0072). (3) miR-25 expression was found to be significantly higher in ESCC tissues (P=0.0157) and ESCC cell lines (P=0.0093) than in normal tissues and fibroblasts. (4) In a large-scale validation analysis, plasma miR-25 levels were significantly higher in 105 preoperative (P<0.0001) ESCC patients who underwent curative oesophagectomy and 20 superficial ESCC patients who underwent endoscopic resection (P<0.0001) than in 50 healthy volunteers. (5) Plasma miR-25 levels were significantly reduced in postoperative samples than in preoperative samples (P<0.0005) and were significantly increased during ESCC recurrences (P=0.0145).
Conclusions:
Plasma miR-25 might be a clinically useful biomarker for cancer detection and the monitoring of tumour dynamics in ESCC patients.
Similar content being viewed by others
Main
Oesophageal carcinoma is the sixth leading cause of cancer-related deaths worldwide (Jemal et al, 2011). Although there are two histological types of oesophageal carcinoma, oesophageal squamous cell carcinoma (ESCC) is the predominant histological type in Asian countries and accounts for approximately 90% of oesophageal carcinomas (Hiyama et al, 2007) and is one of the most aggressive carcinomas of the gastrointestinal tract. Although surgical techniques, perioperative management, and perioperative chemo and/or radiotherapy regimens have greatly progressed, even now ESCC continues to present with an extremely poor prognosis. Therefore, primary tumours must be detected at an early stage and treated with curative intent to improve survival rates, and patients with far advanced disease must be diagnosed preoperatively in order to avoid impairments in their quality of life following unnecessary extended surgery. Moreover, recurrent disease must be diagnosed when it is still minimal or clinically occult to improve the prognosis of ESCC patients.
Because identifying molecular targets for ESCC treatment may contribute to the improvement of survival of patients with this lethal disease, several recent studies have clarified that certain molecules, such as COX-2, BCL-2, TP53, p16, cyclin D1, FAS, EGFR, VEGF, and E-cadherin (Hollstein et al, 1990; Adélaide et al, 1995; Gratas et al, 1998; Meng et al, 2011; Shi et al, 2011), have important roles in tumorigenesis and the development of ESCC. Moreover, various genetic and epigenetic alterations that contribute to the carcinogenesis of ESCC have been elucidated. In clinical settings, however, only a few molecules have been validated as diagnostic, therapeutic, and/or prognostic biomarkers for ESCC. Conventional serum tumour markers, such as carcinoembryonic antigen (CEA) and squamous cell carcinoma antigen (SCC), have been used in convenient diagnostic assays (Kosugi et al, 2004; Mroczko et al, 2008) for the early detection of ESCC and the monitoring of tumour dynamics. These serum tumour markers, however, lack sufficient sensitivity and specificity to enable the early detection of ESCC. Hence, development of novel molecular biomarkers using less invasive technology is necessary and could allow clinicians to detect early ESCC, monitor tumour dynamics, and predict treatment sensitivity and prognosis.
MicroRNAs (miRNAs), which are small non-coding RNAs, regulate the translation of specific protein-coding genes. Since their discovery in 1993 (Lee et al, 1993), miRNAs have been intensively studied in cancer research, and these molecules are predicted to control as much as 30% of all gene expression (Lewis et al, 2005). Thus altered miRNA expression has been associated with several diseases, and tumour miRNAs are involved in tumorigenesis and the development of various cancers (He et al, 2005; Lu et al, 2005; Calin and Croce, 2006; He et al, 2007). Recently, several studies have identified that miRNAs are detectable in plasma/serum (Calin and Croce, 2006; Chen et al, 2008; Filipowicz et al, 2008; Ichikawa et al, 2012). Tumour-derived miRNAs are resistant to endogenous ribonuclease activity, because these may bind to proteins, such as the Argonaute 2 protein and high-density lipoprotein (Arroyo et al, 2011; Vickers et al, 2011), or may be packaged by secretory particles, including apoptotic bodies and exosomes in plasma/serum (Hasselmann et al, 2001; Mitchell et al, 2008; Cocucci et al, 2009; Kosaka et al, 2010). Therefore, miRNAs can be present in a remarkably stable form (Mitchell et al, 2008; Zhu and Fan, 2011), and the expression levels of serum miRNAs are reproducible and consistent among individuals (Chen et al, 2008; Mitchell et al, 2008). Furthermore, secretory vesicles, which include specific miRNAs, can function as intercellular transmitters. Namely, secreted miRNAs from donor cells can be transferred to and function in recipient cells (Valadi et al, 2007; Skog et al, 2008; Rechavi et al, 2009).
Regarding ESCC, several research groups, including our own, have reported the potential utility of circulating miRNAs in plasma/serum in clinical application (Zhang et al, 2010; Komatsu et al, 2011, 2012; Kurashige et al, 2012; Hirajima et al, 2013; Takeshita et al, 2013). Each identified miRNA in the plasma/serum of ESCC patients could be valuable for detecting cancer, monitoring tumour, and predicting prognosis. However, these reported blood-based miRNAs do not always indicate all candidates for ESCC, and more sensitive and promising candidate miRNAs for screening cancer, monitoring the tumour status, and predicting prognosis could be found in clinical settings. Therefore, we performed a genome-wide miRNA array-based approach to detect novel plasma miRNA candidates for detecting ESCC and monitoring the tumour status.
In this study, we selected eight upregulated and common plasma miRNA candidates (miR-15b, 16, 17, 25, 19b, 20a, 20b, and 106a) through the integration of two miRNA array-based approaches, which were used to compare plasma miRNA levels between ESCC patients and healthy volunteers and between preoperative and postoperative ESCC patients, respectively. We validated that plasma miR-25, which is located in the miR-106-25 cluster, is overexpressed in ESCC tissues (Guo et al, 2008; Zhu et al, 2011; Xu et al, 2012; Fang et al, 2013), and has an oncogenic function to negatively regulate tumour suppressive genes such as PTEN, p21, TP53, Bim, CDH1, and DR4 (Petrocca et al, 2008; Kan et al, 2009; Li et al, 2009; Poliseno et al, 2010; Kumar et al, 2011; Razumilava et al, 2012; Xu et al, 2012), was upregulated in ESCC patients and preoperative ESCC patients. Moreover, we clearly demonstrated that plasma miR-25 levels are useful to detect ESCC and monitor tumour dynamics for tumour resection and recurrence. Our results provided evidence that plasma miR-25 levels contribute to clinical decision making in ESCC treatments to a clinically satisfactory degree.
Materials and methods
Patients and samples
This study was approved by the Institutional Review Board of Kyoto Prefectural University of Medicine, and each subject provided written informed consent. Between November 2008 and June 2011, 105 consecutive preoperative plasma samples were collected from consecutive ESCC patients who underwent curative oesophagectomy at the Kyoto Prefectural University of Medicine. Patient characteristics with respect to age, sex, venous invasion, lymphatic invasion, T stage, N stage, disease stage, and resection status are described in Table 1. Moreover, additional 20 consecutive pretreatment plasma samples were collected from consecutive superficial ESCC patients who underwent curative endoscopic resection (m2-sm1, R0). All patients were pathologically diagnosed with ESCC using surgical specimens and biopsies. Ten ESCC specimens were collected from patients undergoing oesophagectomy, and seven normal oesophageal tissue specimens of the abdominal esophagus were collected from patients undergoing total gastrectomy for gastric cancer; these patients were selected for the normal specimens because the non-cancerous oesophageal tissues of ESCC patients may still exhibit dysplasia or potentially be cancerous tissue. As a control, plasma was collected from 50 healthy volunteers. These healthy volunteers included medical personnel and patients with benign diseases, such as cholelithiasis. These patients underwent medical examinations, including endoscopy, and were found not to have any oesophageal disease or other cancerous disease. Tumour stages were assessed according to the Union of International Control of Cancer (UICC) classification (Sobin et al, 2009). The details of plasma samples were summarised in Supplementary Figure S1.
Peripheral blood (7 ml) was obtained from each patient at the time before the oesophagectomy or endoscopic resection and from the healthy volunteers. Blood was collected from patients and controls in sodium heparin tubes (BD Vacutainer, Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and immediately subjected to the three-spin protocol (1500 r.p.m. for 30 min, 3000 r.p.m. for 5 min, and 4500 r.p.m. for 5 min) to prevent contamination by cellular nucleic acids. The plasma samples were stored at −80 °C until further processing. The resected specimens were fixed in formalin and embedded in paraffin for pathological diagnosis. Macroscopic and microscopic classification of tumours was based on the UICC/TMN staging system (Sobin et al, 2009).
RNA extraction
Total RNA was extracted from 400 μl of plasma using the mirVana PARIS Kit (Ambion, Austin, TX, USA) and finally eluted into 100 μl of preheated (95 °C) Elution Solution according to the manufacturer’s protocol. Using the formalin-fixed paraffin-embedded tissues, total RNA was extracted from four 15-μm-thick slices of tissue (total 60 μm in thickness) using the RecoverAll Total Nucleic Acid Isolation Kit (Ambion) and then eluted into 60 μl of Elution Solution according to the manufacturer’s protocol.
miRNA microarray analysis
Microarray analyses of the plasma samples were performed using the 3D-Gene miRNA microarray platform (Toray, Kamakura, Japan) (Nagino et al, 2006; Giovannetti et al, 2012; Konishi et al, 2012). RNA extraction was performed according to the manufacturer’s instructions described previously (Konishi et al, 2012). Briefly, the amount of total RNA in plasma was too small, and so 2 of the 4 μl of extracted total RNA from 300 μl of plasma samples were used in the microarray experiments. The reason why the volume of 300 μl plasma was used as the common denominator in each microarray analysis is that there was no definite internal control in plasma miRNA analyses as shown in our previous studies (Tsujiura et al, 2010; Komatsu et al, 2011; Morimura et al, 2011; Komatsu et al, 2012; Konishi et al, 2012; Hirajima et al, 2013; Kawaguchi et al, 2013). This RNA was labelled with Hy5 using the Label IT miRNA labeling kit (Takara Bio, Otsu, Japan) and hybridised at 32 °C for 16 h on the 3D-Gene chip. The 3D-Gene miRNA microarray (Human_miRNA_17v1.0.0, Toray Industries, Kamakura, Japan) can mount >1500 miRNAs based on the Human miRNA Version17 of MirBase (http://microrna.sanger.ac.uk/). Microarray was scanned, and the obtained images were numerated using the 3D-GeneH scanner 3000 (Toray Industries). The expression level of each miRNA was globally normalised using the background- subtracted signal intensity of the entire miRNAs in each microarray. The obtained microarray images were analysed using GenePix Pro TM (Molecular Device, Sunnyvale, CA, USA).
In these miRNA array-based analyses, two different approaches were performed. One approach was used to compare plasma miRNA levels derived from 300 μl of the plasma sample between ESCC patients and healthy volunteers. Namely, each 100 μl plasma sample from three ESCC patients was equally mixed, and totally 300 μl of plasma sample was used as a sample of ESCC patients. On the other hand, each 60 μl plasma sample from five healthy volunteers was equally mixed, and totally 300 μl of plasma sample was used as a healthy volunteers’ sample. The second approach was used to compare plasma miRNA levels between paired preoperative and postoperative ESCC patients. Each 100 μl plasma sample from three preoperative or postoperative ESCC patients was equally mixed, respectively, and totally 300 μl of a plasma sample was used as a sample of preoperative or postoperative ESCC patients.
Protocol for quantification of miRNA by quantitative RT–PCR (qRT–PCR)
Test-scale analyses were performed using qRT–PCR in order to validate the utility of selected candidates from miRNA array-based approaches. The amounts of miRNAs in the plasma were quantified by qRT–PCR using human TaqMan MicroRNA Assay Kits (Applied Biosystems, Foster City, CA, USA). The reverse transcription reaction was carried out using a TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems) in 15 μl solution containing 5 μl of RNA extract, 0.15 μl of 100 mM dNTPs, 1 μl of MultiScribe reverse transcriptase (50 U μl−1), 1.5 μl of 10 × reverse transcription buffer, 0.19 μl of RNase inhibitor (20 U μl−1), 1 μl of gene-specific primer (has-miR-25, Assay ID: 000403; has-miR-16, Assay ID: 000391; has-miR-20b, Assay ID: 001014; has-miR-106a, Assay ID: 002169; has-miR-17, Assay ID: 002308; has-miR-20a, Assay ID: 000580; has-miR-15b, Assay ID: 000390; has-miR-19b, Assay ID: 000396; and RNU6B, Assay ID: 001093), and 4.16 μl of nuclease-free water. For cDNA synthesis, the reaction mixtures were incubated at 16 °C for 30 min, at 42 °C for 30 min, and at 85 °C for 5 min and then held at 4 °C. Next, 1.33 μl of cDNA solution was amplified using 10 μl of TaqMan 2 × Universal PCR Master Mix with no AmpErase UNG reagent (Applied Biosystems), 1 μl of gene-specific primer/probe, and 7.67 μl of nuclease-free water in a final volume of 20 μl. Quantitative PCR was run on a 7300 Real-time PCR system (Applied Biosystems), and the reaction mixtures were incubated at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. Cycle threshold (Ct) values were calculated using the SDS 1.4 software (Applied Biosystems).
Plasma miRNA levels were calculated on a standard curve constructed using synthetic miRNAs from the mirVana miRNA Reference Panel (Ambion). Standard reference miRNAs were amplified by RT–PCR of a 10-fold serial dilution of the mirVana miRNA Reference Panel. The linearity of quantitative RT–PCR was confirmed between the logarithm of the amount of input miRNA and Ct values for a range of concentrations (1–0.0001 fmol) of each synthetic miRNA.
Although some investigators have determined plasma miRNAs levels by comparing with internal control miRNAs (Ng et al, 2009), it remains controversial as to which miRNAs are suitable as internal controls for plasma assays. Therefore we confirmed a linear correlation between the logarithm of the amount of input synthetic miRNA and the Ct value using real-time PCR, as well as the feasibility of extracting total RNA and amplifying specific miRNA in plasma samples. On the basis of these findings, we utilised the absolute concentration for measuring plasma miRNA in this study.
As the expression of miRNAs from tissue samples was normalised using the 2−ΔΔCT method relative to U6 small nuclear RNA (RNU6B), ΔCt was calculated by subtracting Ct values of RNU6B from those of the miRNAs of interest. ΔΔCt was then calculated by subtracting ΔCt of normal tissue from ΔCt of ESCC tissues. The change in gene expression was calculated using the equation 2−ΔΔCt (Livak and Schmittgen, 2001).
ESCC cell lines and culture
ESCC cell lines such as TE2, TE9, TE13, and KYSE70 and the fibroblast cell line WI-38 were purchased from RIKEN Cell Bank (Tsukuba, Japan). TE2, TE9, TE13, and KYSE70 cells were cultured in Roswell Park Memorial Institute-1640 medium (Sigma, St Louis, MO, USA). WI-38 cells were cultured in Dulbecco’s Minimum Essential Medium. All media were purchased from Sigma and supplemented with 100 ml l−1 fetal bovine serum (Trace Scientific, Melbourne, Victoria, Australia). All cell lines were cultured in 50 ml l−1 carbon dioxide at 37 °C in a humidified chamber.
Statistical analysis
In miRNA array-based analyses, the signal intensity ratio and log2 ratio of each plasma miRNA were calculated by the ratio of ESCC patients/healthy volunteers or pretreatment ESCC patients/postoperative ESCC patents. The Mann–Whitney test was used to compare differences in plasma miRNA levels between the cancer and healthy groups, and the Wilcoxon’s test was used to compare plasma levels between the paired samples before and 1 month after oesophagectomy or between the samples after oesophagectomy and at recurrences. A P-value of 0.05 was considered significant. The area under the receiver operating characteristic (ROC) curve (AUC) was used to assess the feasibility of using plasma miRNA levels as a diagnostic tool for detecting ESCC. The Youden index was used to determine the cutoff value for plasma miRNA levels (Akobeng, 2007).
Results
Study design to detect novel plasma miRNA biomarkers for ESCC
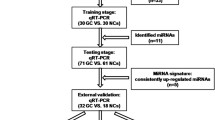
This study was divided into several parts: (1) Selection of miRs that were among the top 15 upregulated miRNAs from two Toray 3D-Gene microRNA array-based approaches in comparisons of ESCC case and control plasmas and preoperative and post-ESCC plasmas, respectively. Of the top 15 miRNAs from each of these comparisons, there were a common set of eight miRNAs that were selected; (2) test-scale analyses using qRT–PCR in order to validate the utility of selected candidates from miRNA array-based approaches, comparing the plasma levels of the eight common miRNAs selected in 20 ESCC patients and 10 healthy controls; (3) evaluation of the correlation between each plasma miRNA level and peripheral blood cells in ESCC patients; (4) large-scale analysis of validation of plasma miR-25 levels by comparing 105 preoperative and 20 superficial ESCC patients who underwent curative oesophagectomy and endoscopic resection, respectively, with 50 healthy volunteers; and (5) evaluation of whether plasma miR-25 levels reflect tumour dynamics in the treatment course of ESCC patients (Figure 1).
Selection of plasma miRNA candidates through the integration of two comprehensive miRNA array-based approaches
Novel miRNA candidates in the plasma for cancer detection and tumour monitoring were selected while using two miRNA array-based approaches to compare plasma miRNA levels between ESCC patients and healthy volunteers (Figure 2A) and between preoperative and postoperative ESCC patients (Figure 2B). Of the 1719 candidate miRNAs analysed, 219 plasma miRNAs were more upregulated in ESCC patients than in healthy volunteers with two-fold changes (Supplementary Table S1), whereas 156 plasma miRNAs were more upregulated in preoperative ESCC patients than in postoperative ESCC patients with two-fold changes (Supplementary Table S2). In order to detect more sensitive biomarkers, we focused on the top 15 miRs in this study (Figure 2). All data were summarised in Supplementary Tables S1 and S2. Of the 15 highly upregulated miRNAs from each group, eight common miRNAs (miR-15b, 16, 17, 25, 19b, 20a, 20b, and 106a) were selected. Concerning miR-451, which we previously reported in gastric cancer detection and monitoring (Konishi et al, 2012), the origin of high miR-451 level in plasma is currently uncertain in cancers, because miR-451 is known as a tumour-suppressive microRNA and downexpressed in primary cancers. (Konishi et al, 2012). Therefore, in this study, we excluded miR-451 for further analyses, because plasma miR-451 needs considerable investigations and is not still suitable for biomarker in cancer detection and tumour monitoring.
Selection of plasma miRNA candidates through the integration of two comprehensive miRNA array-based approaches. While using two miRNA array-based approaches to compare plasma miRNA levels between (A) ESCC patients and healthy volunteers and (B) between preoperative and postoperative ESCC patients, novel miRNA candidates for cancer detection and tumour monitoring were selected. Of the 15 highly upregulated miRNAs from each group, eight common miRNAs were selected.
Test-scale analyses comparing plasma levels of eight common miRNAs in ESCC patients and healthy controls
For test-scale analyses, we investigated the plasma levels of the eight miRNAs selected in 20 ESCC patients and 10 health volunteers by quantitative RT–PCR. miR-25 (P=0.0030) and miR-19b (P=0.0020) were validated to be more significantly upregulated in ESCC patients than in healthy volunteers (Figure 3). miR-106a (P=0.0172) and miR-15b (P=0.0109) were significantly lower in the plasma of ESCC patients than of healthy volunteers. These miRNAs were excluded from further analyses, because their expression patterns were inversely different from those reflected in the array-based results (Figure 2).
Test-scale analyses comparing plasma levels of eight common miRNAs in ESCC patients and healthy controls. For test-scale analyses, we investigated the plasma levels of eight selected miRNAs in 20 ESCC patients and 10 health volunteers by qRT–PCR. miR-25 (P=0.0030) and miR-19b (P=0.0020) were validated to be significantly upregulated in ESCC patients than in healthy volunteers.
Evaluation of the correlation between plasma miRNA levels and peripheral blood cells
Recent reports demonstrated that some circulating miRNAs may be derived from peripheral blood cells (Pritchard et al, 2012; Cheng et al, 2013). We determined the correlation between the plasma levels of the miRNAs selected and peripheral blood cells in 20 ESCC patients. We found a significant correlation between the plasma miR-19b levels and concentrations of red blood cells and haemoglobin in the peripheral blood (Figure 4A). Considering the influence of secretion of miRNAs from blood cells or haemolysis on the results of clinical application, we excluded miR-19b from further analyses in this study. As no significant correlation was observed between plasma miR-25 levels and any type of blood cell in the peripheral blood of 20 ESCC patients,we did not eliminate this miRNA from further analyses (Figure 4B).
Evaluation of the correlation between plasma miRNA levels and peripheral blood cells. The correlation between selected plasma miRNA levels and peripheral blood cells in 20 ESCC patients was determined. (A) A significant correlation was observed between plasma miR-19b levels and concentrations of red blood cells and haemoglobin in the peripheral blood, (B) whereas no significant correlation was observed between plasma miR-25 levels and any type of blood cells in the peripheral blood.
Large-scale analysis of validation of plasma miR-25 levels by comparing ESCC patients with healthy volunteers
Next, plasma miR-25 levels were examined on a large scale for our validation study using plasma samples of ESCC patients and healthy volunteers in qRT–PCR assays. The linearity of qRT–PCR was confirmed from concentrations of 1 fmol to 0.0001 fmol of each synthetic miRNA, such as miR-25 (R2=0.9934) between the logarithm of the amount of input miRNA and Ct values (Supplementary Figure S2). Plasma miR-25 was detectable in all samples from 105 consecutive ESCC patients and 50 healthy volunteers. The differential expression of plasma miR-25 in the ESCC patients was compared with that in normal healthy volunteers using a waterfall plot (Figure 5C). Plasma miR-25 levels were significantly higher in the ESCC patients than in the healthy volunteers (P<0.0001; ESCC patients vs healthy volunteers (mean±s.d.): 0.51±0.57 vs 0.19±0.23 amol μl−1) (Figure 5C). No significant correlation was observed between the plasma miR-25 levels and any type of blood cell in the peripheral blood of the 105 ESCC patients (Supplementary Figure S3). Table 1 shows the association between the plasma miR-25 levels and clinicopathological factors in the 105 consecutive ESCC patients. A high plasma miR-25 level was shown to be significantly associated with the absence of lymphatic invasion (P=0.0110) and early tumour stage in ESCC (P=0.0115).
Evaluation of miR-25 levels in ESCC tissues, ESCC cell lines and plasma samples of patients with ESCC. (A) Difference in miR-25 expression between ESCC tissues and normal tissues determined by a waterfall plot. miR-25 expression levels were significantly higher in ESCC tissues than in normal oesophageal tissues (P=0.0157). The upper and lower limits of the boxes and lines inside the boxes indicate the 75th and 25th percentiles and the median, respectively. The upper and lower horizontal bars denote the 90th and 10th percentiles, respectively. (B) Difference in miR-25 expression between ESCC cell lines, a fibroblast cell line WI-38, and normal tissues. miR-25 expression levels were significantly higher in ESCC cell lines than in both a fibroblast cell line and normal oesophageal tissues (P=0.0093). (C) Plasma miR-25 levels in 105 consecutive ESCC patients and 50 healthy volunteers. Using a real-time RT–PCR assay, difference in plasma miR-25 expression between ESCC patients and normal healthy volunteers was determined by a waterfall plot. Plasma miR-25 levels were significantly higher in ESCC patients than in healthy volunteers (P<0.0001).
Further study on clinical application of plasma miR-25 in superficial ESCC patients undergoing endoscopic resection
To further evaluate the possibility of clinical application of plasma miR-25, plasma miR-25 levels in 20 consecutive superficial ESCC patients who underwent curative endoscopic resection (m2-sm1, R0) and 50 healthy volunteers were examined by real-time PCR (Figure 6A). The plasma miR-25 levels were significantly higher in the superficial ESCC patients than in the healthy volunteers (mean±s.d.: 1.82±0.54 vs 0.19±0.23, P<0.0001). Figure 6B shows that the value for the AUC for the plasma miR-25 analysis was 0.8555. To detect any cutoff points that could differentiate cancer patients from healthy volunteers, we utilised the AUC with the Youden index (Akobeng, 2007). An optimal cutoff point was indicated at 0.32 amol μl−1, with a sensitivity of 85.0% and a specificity of 86.0%. Indeed, the sensitivity of plasma miR-25 for superficial ESCC detection was much higher than that of conventional tumour markers, including SCC, CEA, and CYFRA, at our institute (pT0-1; SCC, CEA, and CYFRA: 25.0, 21.1, and 12.5%, respectively) (details not shown). Our results provided evidence that plasma miR-25 levels can be used to distinguish early ESCC patients from healthy volunteers to a clinically satisfactory degree in comparison with conventional tumour markers.
Plasma miR-25 levels in 20 consecutive superficial ESCC patients undergoing curative endoscopic resection and 50 healthy volunteers. (A) Plasma miR-25 levels were significantly higher in superficial ESCC patients than in healthy volunteers (P<0.0001). (B) The area under the receiver operating characteristic curve was 0.8555.
Evaluation to determine whether plasma miR-25 levels reflect tumour dynamics in the treatment course of ESCC patients
To validate whether plasma miR-25 levels reflect tumour dynamics, we first confirmed the higher miR-25 expression in primary ESCC tissues and ESCC cell lines. miR-25 expression in 10 ESCC tissues and 7 normal oesophageal tissues was determined by qRT–PCR. Moreover, the human ESCC cell lines TE-2, TE-9, TE-13, and KYSE70, and a human fibroblast cell line WI-38 were evaluated by qRT–PCR. The results are shown in Figure 5A and B after normalisation to control U6 expression. miR-25 levels were significantly higher in ESCC tissues than in normal ESCC tissues (P=0.0157). Furthermore, the miR-25 levels were significantly higher in the ESCC cell lines than in the fibroblast cell line and the normal oesophageal tissues (P=0.0093). Consequently, miR-18a expression was shown to be significantly increased in most ESCC tissues and cell lines but not in normal tissues and fibroblasts. Second, the miR-25 level was analysed in paired preoperative and postoperative plasma samples from ESCC patients who underwent curative oesophagectomy and endoscopic resection. As shown in the array-based approach (Figure 2B), plasma miR-25 levels were shown to be significantly reduced in samples after oesophagectomy (P=0.0001) (Figure 7A). In some patients from whom paired samples were collected after oesophagectomy and after recurrences, a significant re-elevation in the plasma miR-25 levels was found at recurrence (P=0.0145) (Figure 7B). These findings indicated that the plasma miR-25 level might reflect tumour dynamics in the treatment course of ESCC patients.
Evaluation of whether plasma miR-25 level could reflect tumor dynamics. (A) Comparison of plasma miR-25 concentrations between preoperative and postoperative samples from ESCC patients. Plasma miR-25 levels were significantly lower in postoperative samples than in preoperative samples (P=0.0001). (B) Comparison of plasma miR-25 levels between postoperative and recurrence state samples from ESCC patients. In some patients from whom paired samples were collected after oesophagectomy and after recurrences, a significant re-elevation in plasma miR-25 levels was found at recurrence (P=0.0145).
Discussion
During the course of a programme of genome-wide miRNA profiling in the plasma of ESCC patients using high-resolution miRNA arrays, we identified a novel plasma miR-25. The plasma expression of this miRNA was significantly higher in preoperative ESCC patients and in pretreatment superficial ESCC patients undergoing endoscopic resection than in postoperative ESCC patients and healthy volunteers. Moreover, plasma miR-25 expression was independent of the blood cell status and reflects the tumour dynamics of preoperation and postoperation and recurrences. Our results provide evidence that plasma miR-25 levels contribute to cancer detection and can be used to monitor ESCC patients to a clinically satisfactory degree in comparison with conventional tumour markers.
During the past decade, non-coding RNAs, so-called miRNAs, have been demonstrated to regulate gene expression by targeting mRNAs for translational repression or cleavage. Moreover, miRNAs have emerged as integral components of the oncogenic and tumour-suppressor network, regulating almost all cellular processes altered during tumour development and may provide new therapeutic strategies such as biomarkers and therapeutic targets for cancers (He et al, 2005; Lu et al, 2005; Calin and Croce, 2006; He et al, 2007). Furthermore, miRNAs have been shown to be present in a remarkably stable form in plasma/serum, and the expression level of serum/plasma miRNAs is reproducible and consistent among individuals (Chen et al, 2008; Mitchell et al, 2008). In fact, a number of reports have suggested the presence of circulating miRNAs and their potential use as novel biomarkers for cancers, such as prostate cancer (Mitchell et al, 2008), leukaemia (Lawrie et al, 2008), oral cancer (Wong et al, 2008), pancreatic cancer (Wang et al, 2009; Morimura et al, 2011; Kawaguchi et al, 2013), colorectal cancer (Ng et al, 2009), lung cancer (Hu et al, 2010), breast cancer (Heneghan et al, 2010), and gastric cancer (Tsujiura et al, 2010; Konishi et al, 2012).
In ESCC, there have been only a few reports, including three reports from our research group, on the role of circulating miRNAs in plasma/serum patients with ESCC (Zhang et al, 2010; Komatsu et al, 2011, 2012; Kurashige et al, 2012; Hirajima et al, 2013; Takeshita et al, 2013). However, these reports did not always indicate that these reported miRNAs were the only candidate biomarkers for ESCC. Therefore more sensitive and promising candidate miRNAs for screening cancer, monitoring the tumour status, and predicting prognosis need to be found in clinical settings. There have been only two specific reports about serum miRNA using the comprehensive array-based approach. Namely, Zhang et al (2010) investigated the serum miRNA profiles in ESCC patients using Solexa sequencing. Among the 25 selected candidate miRNAs analysed, they identified 7 serum miRNAs (miR-10a, 22, 100, 148b, 223, 133a, and 127-3p) as ESCC biomarkers (Zhang et al, 2010). Moreover, Takeshita et al (2013) examined serum miRNA profiles using Agilent human miRNA microarrays to compare the serum miRNA levels in ESCC patients with the levels in healthy controls. Thus miR-1246 was the most markedly elevated miRNA in ESCC patients and was proved to be an independent poor prognostic factor (Takeshita et al, 2013). However, there have been no reported miRNAs in the plasma of ESCC patients that had been identified by the comprehensive array-based approach. Previous studies about miRNA profiles revealed that the majority of circulating miRNAs were co-fractionated with plasma protein complexes (Arroyo et al, 2011). Moreover, plasma might include more abundant protein levels, including cogulant-related proteins, than serum. Indeed, the detected candidate miRNAs were considerably different, because the profiles of cancer-associated miRNAs might be different between plasma and serum (Takeshita et al, 2013). These findings prompted us to further search for novel cancer-associated miRNAs in plasma through a comprehensive miRNA microarray-based approach.
In this study, we finally identified a novel ESCC screening and monitoring plasma miRNA, miR-25, which is included in the miR-106b-25 cluster comprising miR-106b, miR-93, and miR-25 (Yu et al, 2006) and is located within the intron13 of MCM7 gene locus at 7q23.1. The region for these miRNAs was reported to be frequently overexpressed/amplified in ESCC tissue and cell lines (Shinomiya et al, 1999; Guo et al, 2008; Sakai et al, 2010; Zhu et al, 2011; Xu et al, 2012). This genomic aberration of the miR-106b-25 cluster was also accumulated in various other cancers, including gastric cancer (Petrocca et al, 2008), hepatocellular carcinoma (Li et al, 2009), prostate cancer (Poliseno et al, 2010), multiple myeloma (Pichiorri et al, 2008), ovarian cancer (Zhang et al, 2012), and breast cancer (Wu et al, 2012). Furthermore, several recent studies have demonstrated that the miR-106b-25 cluster and its individual miRNAs have pro-oncogenic functions, including mediating pro-proliferative and antiapoptotic phenotypes (Ren et al, 2006; Petrocca et al, 2008; Kan et al, 2009; Li et al, 2009; Poliseno et al, 2010; Martens-Uzunova et al, 2011). Some of the identified crucial functions of miR-106b-25 are the inhibition of tumour-suppressor genes such as PTEN (Poliseno et al, 2010), p21/WAF1 and Bim (Petrocca et al, 2008; Kan et al, 2009; Li et al, 2009), TP53 (Kumar et al, 2011), CDH1 (Xu et al, 2012), and DR4 (Razumilava et al, 2012) in several cancers and the activation of E2F1, which leads to impaired TGF-β signalling and to proliferation, dysregulated cell cycling, and increased invasiveness in HNSCC (Hui et al, 2010). Furthermore, these oncogenic miRNAs in the miR-106b-25 cluster have been shown to be upregulated in cancer stromal tissues compared with normal stroma (Nishida et al, 2012). These findings strongly suggested that miR-25 may have a pivotal role in carcinogenesis and the development of tumours and that upregulation of plasma miR-25 might be associated with the molecular behaviours of ESCC. Indeed, a high plasma miR-25 level was proved to be significantly associated with early tumour stage in ESCC (P=0.0115), and in superficial ESCC patients undergoing endoscopic resection, the value for the AUC, which was used to assess the feasibility of using plasma miRNA levels as a diagnostic tool for the early detection of ESCC, was great (0.8555). This suggests that miR-25 has an important role in the early phase of cancer development. Thus plasma miR-25 levels may be a useful biomarker for the early detection of ESCC.
To further evaluate the clinical possibility of miR-25 for monitoring tumour dynamics, we investigated whether plasma miR-25 levels could reflect tumour dynamics in ESCC by three different analyses. The first analysis was the confirmation of comparative higher miR-25 expression in primary ESCC tissues and ESCC cell lines than in normal oesophageal tissues and fibroblast cells. The second analysis was to confirm that the miR-25 levels were significantly reduced after curative oesophagectomy and endoscopic resection in patients with high preoperative plasma miR-25 levels (Figure 7A). The third analysis was to determine that the significant re-elevation in the plasma miR-25 levels was found at recurrences after curative oesophagectomy or endoscopic resection (Figure 7B). These findings were similar to those of our previous reports (Tsujiura et al, 2010; Komatsu et al, 2011; Morimura et al, 2011; Konishi et al, 2012; Hirajima et al, 2013; Kawaguchi et al, 2013) and clearly demonstrated that plasma miR-25 levels reflected tumour dynamics and may be available as a new plasma biomarker for monitoring the tumour status of ESCC patients.
Recently, Pritchard and his colleagues reported a caution in a cancer biomarker study of circulating miRNAs because the circulating miRNAs might have been derived from peripheral blood cells (Pritchard et al, 2012; Cheng et al, 2013). The specific miRNAs associated with haemolysis and the secretion of miRNAs from blood cells might affect their plasma levels in clinical application. Therefore we evaluated the correlation between both miR-25 and miR-19 levels in plasma and peripheral blood cells in both 20 ESCC patients as a test study and in 105 consecutive ESCC patients as a validation cohort study. A significant association was observed between the plasma miR-19 levels and concentrations of red blood cells and haemoglobin in the peripheral blood (Figure 4A). However, as for miR-25, no association was found between them in both the 20 ESCC patients as a test study and the 105 consecutive ESCC patients as a validation cohort study (Figure 4B, Supplementary Figure S3). These results might exclude the possibility that the secretion of miRNAs from blood cells or haemolysis affected the miR-25 level in clinical settings. However, because secretion, kinetics, and metabolism in plasma miRNAs have not been clearly elucidated, this issue is currently under evaluation.
Taken together, through the integration of two comprehensive miRNA array-based approaches, we clearly demonstrated that plasma miR-25 levels may potentially be useful for screening cancer and monitoring tumour dynamics in ESCC patients. Nevertheless, many issues must be addressed before these findings can be translated into a clinically useful, non-invasive screening strategy for ESCC patients. Therefore we will prospectively confirm the usefulness of plasma miR-25 in a large number of patients. Furthermore, we believe that more sensitive plasma miRNA biomarkers could be identified by different methods such as a recent next-generation sequence or digital PCR-based approach and could translate circulating miRNAs into the clinical setting. These strategies are currently under evaluation, and we will report in the near future.
Change history
14 October 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Adélaide J, Monges G, Dérdérian C, Seitz JF, Birnbaum D (1995) Oesophageal cancer and amplification of the human cyclin D gene CCND1/PRAD1. Br J Cancer 71: 64–68.
Akobeng AK (2007) Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr 96: 644–647.
Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M (2011) Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA 108: 5003–5008.
Calin GA, Croce CM (2006) MicroRNA signatures in human cancers. Nat Rev Cancer 6: 857–866.
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Zen K, Zhang CY (2008) Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 18: 997–1006.
Cheng HH, Yi HS, Kim Y, Kroh EM, Chien JW, Eaton KD, Goodman MT, Tait JF, Tewari M, Pritchard CC (2013) Plasma processing conditions substantially influence circulating microRNA biomarker levels. PLoS One 8: e64795.
Cocucci E, Racchetti G, Meldolesi J (2009) Shedding microvesicles: artefacts no more. Trends Cell Biol 19: 43–41.
Fang WK, Liao LD, Li LY, Xie YM, Xu XE, Zhao WJ, Wu JY, Zhu MX, Wu ZY, Du ZP, Wu BL, Xie D, Guo MZ, Xu LY, Li EM (2013) Down-regulated desmocollin-2 promotes cell aggressiveness through redistributing adherens junctions and activating beta-catenin signalling in oesophageal squamous cell carcinoma. J Pathol 231: 257–270.
Filipowicz W, Bhattacharyya SN, Sonenberg N (2008) Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet 9: 102–114.
Giovannetti E, van der Velde A, Funel N, Vasile E, Perrone V, Leon LG, De Lio N, Avan A, Caponi S, Pollina LE, Gallá V, Sudo H, Falcone A, Campani D, Boggi U, Peters GJ (2012) High-throughput microRNA (miRNAs) arrays unravel the prognostic role of MiR-211 in pancreatic cancer. PLoS One 7: e49145.
Gratas C, Tohma Y, Barnas C, Taniere P, Hainaut P, Ohgaki H (1998) Up-regulation of Fas (APO-1/CD95) ligand and down-regulation of Fas expression in human esophageal cancer. Cancer Res 58: 2057–2062.
Guo Y, Chen Z, Zhang L, Zhou F, Shi S, Feng X, Li B, Meng X, Ma X, Luo M, Shao K, Li N, Qiu B, Mitchelson K, Cheng J, He J (2008) Distinctive microRNA profiles relating to patient survival in esophageal squamous cell carcinoma. Cancer Res 68: 26–33.
Hasselmann DO, Rappl G, Tilgen W, Reinhold U (2001) Extracellular tyrosinase mRNA within apoptotic bodies is protected from degradation in human serum. Clin Chem 47: 1488–1489.
He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ (2007) A microRNA component of the p53 tumour suppressor network. Nature 447: 1130–1134.
He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, Hammond SM (2005) A microRNA polycistron as a potential human oncogene. Nature 435: 828–833.
Heneghan HM, Miller N, Lowery AJ, Sweeney KJ, Newell J, Kerin MJ (2010) Circulating microRNAs as novel minimally invasive biomarkers for breast cancer. Ann Surg 251: 499–505.
Hirajima S, Komatsu S, Ichikawa D, Takeshita H, Konishi H, Shiozaki A, Morimura R, Tsujiura M, Nagata H, Kawaguchi T, Arita T, Kubota T, Fujiwara H, Okamoto K, Otsuji E (2013) Clinical impact of circulating miR-18a in plasma of patients with oesophageal squamous cell carcinoma. Br J Cancer 108: 1822–1829.
Hiyama T, Yoshihara M, Tanaka S, Chayama K (2007) Genetic polymorphisms and esophageal cancer risk. Int J Cancer 121: 1643–1658.
Hollstein MC, Metcalf RA, Welsh JA, Montesano R, Harris CC (1990) Frequent mutation of the p53 gene in human esophageal cancer. Proc Natl Acad Sci USA 87: 9958–9961.
Hu Z, Chen X, Zhao Y, Tian T, Jin G, Shu Y, Chen Y, Xu L, Zen K, Zhang C, Shen H (2010) Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J Clin Oncol 28: 1721–1726.
Hui AB, Lenarduzzi M, Krushel T, Waldron L, Pintilie M, Shi W, Perez-Ordonez B, Jurisica I, O’Sullivan B, Waldron J, Gullane P, Cummings B, Liu FF (2010) Comprehensive MicroRNA profiling for head and neck squamous cell carcinomas. Clin Cancer Res 16: 1129–1139.
Ichikawa D, Komatsu S, Konishi H, Otsuji E (2012) Circulating microRNA in digestive tract cancers. Gastroenterology 142: 1074–1078.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90.
Kan T, Sato F, Ito T, Matsumura N, David S, Cheng Y, Agarwal R, Paun BC, Jin Z, Olaru AV, Selaru FM, Hamilton JP, Yang J, Abraham JM, Mori Y, Meltzer SJ (2009) The miR-106b-25 polycistron, activated by genomic amplification, functions as an oncogene by suppressing p21 and Bim. Gastroenterology 136: 1689–1700.
Kawaguchi T, Komatsu S, Ichikawa D, Morimura R, Tsujiura M, Konishi H, Takeshita H, Nagata H, Arita T, Hirajima S, Shiozaki A, Ikoma H, Okamoto K, Ochiai T, Taniguchi H, Otsuji E (2013) Clinical impact of circulating miR-221 in plasma of patients with pancreatic cancer. Br J Cancer 108: 361–369.
Komatsu S, Ichikawa D, Takeshita H, Konishi H, Nagata H, Hirajima S, Kawaguchi T, Arita T, Shiozaki A, Fujiwara H, Okamoto K, Otsuji E (2012) Prognostic impact of circulating miR-21 and miR-375 in plasma of patients with esophageal squamous cell carcinoma. Expert Opin Biol Ther 12 (Suppl 1): S53–S59.
Komatsu S, Ichikawa D, Takeshita H, Tsujiura M, Morimura R, Nagata H, Kosuga T, Iitaka D, Konishi H, Shiozaki A, Fujiwara H, Okamoto K, Otsuji E (2011) Circulating microRNAs in plasma of patients with oesophageal squamous cell carcinoma. Br J Cancer 105: 104–111.
Konishi H, Ichikawa D, Komatsu S, Shiozaki A, Tsujiura M, Takeshita H, Morimura R, Nagata H, Arita T, Kawaguchi T, Hirashima S, Fujiwara H, Okamoto K, Otsuji E (2012) Detection of gastric cancer-associated microRNAs on microRNA microarray comparing pre- and post-operative plasma. Br J Cancer 106: 740–747.
Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T (2010) Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 285: 17442–17452.
Kosugi S, Nishimaki T, Kanda T, Nakagawa S, Ohashi M, Hatakeyama K (2004) Clinical significance of serum carcinoembryonic antigen, carbohydrate antigen 19-9, and squamous cell carcinoma antigen levels in esophageal cancer patients. World J Surg 28: 680–685.
Kumar M, Lu Z, Takwi AA, Chen W, Callander NS, Ramos KS, Young KH, Li Y (2011) Negative regulation of the tumor suppressor p53 gene by microRNAs. Oncogene 30: 843–853.
Kurashige J, Kamohara H, Watanabe M, Tanaka Y, Kinoshita K, Saito S, Hiyoshi Y, Iwatsuki M, Baba Y, Baba H (2012) Serum microRNA-21 is a novel biomarker in patients with esophageal squamous cell carcinoma. J Surg Oncol 106: 188–192.
Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, Banham AH, Pezzella F, Boultwood J, Wainscoat JS, Hatton CS, Harris AL (2008) Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol 141: 672–675.
Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75: 843–854.
Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20.
Li Y, Tan W, Neo TW, Aung MO, Wasser S, Lim SG, Tan TM (2009) Role of the miR-106b-25 microRNA cluster in hepatocellular carcinoma. Cancer Sci 100: 1234–1242.
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408.
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR (2005) MicroRNA expression profiles classify human cancers. Nature 435: 834–838.
Martens-Uzunova ES, Jalava SE, Dits NF, van Leenders GJ, Møller S, Trapman J, Bangma CH, Litman T, Visakorpi T, Jenster G (2011) Diagnostic and prognostic signatures from the small non-coding RNA transcriptome in prostate cancer. Oncogene 31: 978–991.
Meng XY, Zhu ST, Zong Y, Wang YJ, Li P, Zhang ST (2011) Promoter hypermethylation of cyclooxygenase-2 gene in esophageal squamous cell carcinoma. Dis Esophagus 24: 444–449.
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M (2008) Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 105: 10513–10518.
Morimura R, Komatsu S, Ichikawa D, Takeshita H, Tujiura M, Nagata H, Konishi H, Shiozaki A, Ikoma H, Okamoto K, Ochiai T, Taniguchi H, Otsuji E (2011) Novel diagnostic value of circulating miR-18a in plasma of patients with pancreatic cancer. Br J Cancer 11: 1733–1740.
Mroczko B, Kozłowski M, Groblewska M, Łukaszewicz M, Nikliński J, Jelski W, Laudański J, Chyczewski L, Szmitkowski M (2008) The diagnostic value of the measurement of matrix metalloproteinase 9 (MMP-9), squamous cell cancer antigen (SCC) and carcinoembryonic antigen (CEA) in the sera of esophageal cancer patients. Clin Chim Acta 389: 61–66.
Nagino K, Nomura O, Takii Y, Myomoto A, Ichikawa M, Nakamura F, Higasa M, Akiyama H, Nobumasa H, Shiojima S, Tsujimoto G (2006) Ultrasensitive DNA chip: gene expression profile analysis without RNA amplification. J Biochem 139: 697–703.
Ng EK, Chong WW, Jin H, Lam EK, Shin VY, Yu J, Poon TC, Ng SS, Sung JJ (2009) Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut 58: 1375–1381.
Nishida N, Nagahara M, Sato T, Mimori K, Sudo T, Tanaka F, Shibata K, Ishii H, Sugihara K, Doki Y, Mori M (2012) Microarray analysis of colorectal cancer stromal tissue reveals upregulation of two oncogenic miRNA clusters. Clin Cancer Res 18: 3054–3070.
Petrocca F, Visone R, Onelli MR, Shah MH, Nicoloso MS, de Martino I, Iliopoulos D, Pilozzi E, Liu CG, Negrini M, Cavazzini L, Volinia S, Alder H, Ruco LP, Baldassarre G, Croce CM, Vecchione A (2008) E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell 13: 272–286.
Pichiorri F, Suh SS, Ladetto M, Kuehl M, Palumbo T, Drandi D, Taccioli C, Zanesi N, Alder H, Hagan JP, Munker R, Volinia S, Boccadoro M, Garzon R, Palumbo A, Aqeilan RI, Croce CM (2008) MicroRNAs regulate critical genes associated with multiple myeloma pathogenesis. Proc Natl Acad Sci USA 105: 12885–12890.
Poliseno L, Salmena L, Riccardi L, Fornari A, Song MS, Hobbs RM, Sportoletti P, Varmeh S, Egia A, Fedele G, Rameh L, Loda M, Pandolfi PP (2010) Identification of the miR-106b∼25 microRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates with its host gene MCM7 in transformation. Sci Signal 3: ra29 Erratum in: Sci Signal. 2010;3(123):er6.
Pritchard CC, Kroh E, Wood B, Arroyo JD, Dougherty KJ, Miyaji MM, Tait JF, Tewari M (2012) Blood cell origin of circulating microRNAs: a cautionary note for cancer biomarker studies. Cancer Prev Res (Phila) 5: 492–497.
Razumilava N, Bronk SF, Smoot RL, Fingas CD, Werneburg NW, Roberts LR, Mott JL (2012) miR-25 targets TNF-related apoptosis inducing ligand (TRAIL) death receptor-4 and promotes apoptosis resistance in cholangiocarcinoma. Hepatology 55: 465–475.
Rechavi O, Erlich Y, Amram H, Flomenblit L, Karginov FV, Goldstein I, Hannon GJ, Kloog Y (2009) Cell contact-dependent acquisition of cellular and viral nonautonomously encoded small RNAs. Genes Dev 23: 1971–1979.
Ren B, Yu G, Tseng GC, Cieply K, Gavel T, Nelson J, Michalopoulos G, Yu YP, Luo JH (2006) MCM7 amplification and overexpression are associated with prostate cancer progression. Oncogene 25: 1090–1098.
Sakai N, Kajiyama Y, Iwanuma Y, Tomita N, Amano T, Isayama F, Ouchi K, Tsurumaru M (2010) Study of abnormal chromosome regions in esophageal squamous cell carcinoma by comparative genomic hybridization: relationship of lymph node metastasis and distant metastasis to selected abnormal regions. Dis Esophagus 23: 415–421.
Shi HY, Lv FJ, Zhu ST, Wang QG, Zhang ST (2011) Dual inhibition of 5-LOX and COX-2 suppresses esophageal squamous cell carcinoma. Cancer Lett 309: 19–26.
Shinomiya T, Mori T, Ariyama Y, Sakabe T, Fukuda Y, Murakami Y, Nakamura Y, Inazawa J (1999) Comparative genomic hybridization of squamous cell carcinoma of the esophagus: the possible involvement of the DPI gene in the 13q34 amplicon. Genes Chromosomes Cancer 24: 337–344.
Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10: 1470–1476.
Sobin LH, Gospodarowicz MK, Wittekind C (2009) TNM Classification of Malignant Tumors 7th edn. Wiley-liss: New York, NY, USA.
Takeshita N, Hoshino I, Mori M, Akutsu Y, Hanari N, Yoneyama Y, Ikeda N, Isozaki Y, Maruyama T, Akanuma N, Komatsu A, Jitsukawa M, Matsubara H (2013) Serum microRNA expression profile: miR-1246 as a novel diagnostic and prognostic biomarker for oesophageal squamous cell carcinoma. Br J Cancer 108: 644–652.
Tsujiura M, Ichikawa D, Komatsu S, Shiozaki A, Takeshita H, Kosuga T, Konishi H, Morimura R, Deguchi K, Fujiwara H, Okamoto K, Otsuji E (2010) Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer 102: 1174–1179.
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659.
Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT (2011) MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 13: 423–433.
Wang J, Chen J, Chang P, LeBlanc A, Li D, Abbruzzesse JL, Frazier ML, Killary AM, Sen S (2009) MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev Res (Phila) 2: 807–813.
Wong TS, Liu XB, Wong BY, Ng RW, Yuen AP, Wei WI (2008) Mature miR-184 as potential oncogenic microRNA of squamous cell carcinoma of tongue. Clin Cancer Res 14: 2588–2592.
Wu Q, Wang C, Lu Z, Guo L, Ge Q (2012) Analysis of serum genome-wide microRNAs for breast cancer detection. Clin Chim Acta 413: 1058–1065.
Xu X, Chen Z, Zhao X, Wang J, Ding D, Wang Z, Tan F, Tan X, Zhou F, Sun J, Sun N, Gao Y, Shao K, Li N, Qiu B, He J (2012) MicroRNA-25 promotes cell migration and invasion in esophageal squamous cell carcinoma. Biochem Biophys Res Commun 421: 640–645.
Yu J, Wang F, Yang GH, Wang FL, Ma YN, Du ZW, Zhang JW (2006) Human microRNA clusters: genomic organization and expression profile in leukemia cell lines. Biochem Biophys Res Commun 349: 59–68.
Zhang C, Wang C, Chen X, Yang C, Li K, Wang J, Dai J, Hu Z, Zhou X, Chen L, Zhang Y, Li Y, Qiu H, Xing J, Liang Z, Ren B, Yang C, Zen K, Zhang CY (2010) Expression profile of microRNAs in serum: a fingerprint for esophageal squamous cell carcinoma. Clin Chem 56: 1871–1879.
Zhang H, Zuo Z, Lu X, Wang L, Wang H, Zhu Z (2012) MiR-25 regulates apoptosis by targeting Bim in human ovarian cancer. Oncol Rep 27: 594–598.
Zhu H, Fan GC (2011) Extracellular/circulating microRNAs and their potential role in cardiovascular disease. Am J Cardiovasc Dis 2: 138–149.
Zhu L, Yan W, Rodriguez-Canales J, Rosenberg AM, Hu N, Goldstein AM, Taylor PR, Erickson HS, Emmert-Buck MR, Tangrea MA (2011) MicroRNA analysis of microdissected normal squamous esophageal epithelium and tumor cells. Am J Cancer Res 1: 574–584.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Komatsu, S., Ichikawa, D., Hirajima, S. et al. Plasma microRNA profiles: identification of miR-25 as a novel diagnostic and monitoring biomarker in oesophageal squamous cell carcinoma. Br J Cancer 111, 1614–1624 (2014). https://doi.org/10.1038/bjc.2014.451
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2014.451
- Springer Nature Limited
Keywords
This article is cited by
-
Low blood level of tumour suppressor miR-5193 as a target of immunotherapy to PD-L1 in gastric cancer
British Journal of Cancer (2024)
-
Loss of miR-637 promotes cancer cell stemness via WASH/IL-8 pathway and serves as a novel prognostic marker in esophageal squamous cell carcinoma
Biomarker Research (2022)
-
A microRNA-based liquid biopsy signature for the early detection of esophageal squamous cell carcinoma: a retrospective, prospective and multicenter study
Molecular Cancer (2022)
-
miRNA regulation of G protein-coupled receptor mediated angiogenic pathways in cancer
The Nucleus (2021)
-
Integrated analysis of genome-wide miRNAs and targeted gene expression in esophageal squamous cell carcinoma (ESCC) and relation to prognosis
BMC Cancer (2020)