Abstract
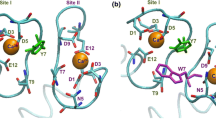
Calcium performs a unique role in biology, achieving biological effects through highly specific interactions with and modulation of target proteins1. It has been proposed that calcium-modulated proteins possess a characteristic, evolutionarily related, binding fold, known as the EF-hand2. The high-resolution X-ray structure of α-lactalbumin reveals a Ca2+ binding fold that resembles an EF-hand only superficially and presumably has no evolutionary relationship with it. However, there is clear homology with the corresponding loop in c-type lysozyme (the ‘parent’ molecule of α-lactalbumin). This study, at 1.7 Å resolution, represents one of the most accurate analyses of a calcium binding protein yet reported.
Similar content being viewed by others
References
Levine, B. A. & Williams, R. J. P. in Calcium and Cell Function vol. II, (ed. Cheung, W.Y.) 1–38 (Academic, New York, 1982).
Kretsinger, R. H. & Nockolds, C. E. J. biol. Chem. 248, 3313–3326 (1973).
Brew, K. & Campbell, P. N. Biochem. J. 102, 258–269 1967).
Aschaffenburg, R. & Drewry, J. Biochem. J. 65, 273–277 (1957).
Qasba, P. K. & Safaya, S. K. Nature 308, 377–380 (1984).
Hiraoka, Y. et al. Biochem. biophys. Res. Commun., 95, 1098–1104 (1980).
Mitani, M., et al. J. biol. Chem., 261, 8824–8829 (1986).
Musci, G., & Berliner, L. J. Biochemistry 24, 6945–6948. 1985).
Hiraoka, Y. & Sugai, S. Int. J. Pept. Prot. Res. 23, 535–542 (1984).
Herzberg, O., Moult, J. & James, M. N. G. J. biol. Chem. 261, 2638–2644 (1986).
Aschaffenburg, R. et al. J. molec. Biol., 67, 525–528 (1972).
Fenna, R. E. J. molec. Biol., 161, 203–210 (1982).
Aschaffenburg, R. et al. J. molec. Biol. 127, 135–137 (1979).
Smith, S. G. et al. Biochem. J. (submitted).
Hall, L. et al. Nucleic Acid Res. 10, 3503–3515 (1982).
Shewale J. G., Sinha, S. K. & Bew K. J. biol. Chem. 259, 4947–4956 1984).
Kuwajima, K., Hiroshima, Y. & Sugai, S. Int. J. Pept. Prot. Res. 27, 18–27 (1986).
Herzberg, O., & James, M. N. G. Biochemistry 24, 5298–5302 (1985).
Rees, D. C. Lewis, M. & Lipscomb, W. N. J. molec. Biol. 168, 367–387 (1983).
Jones, T. A. & Thirup, S. EMBO J. 5, 819–822 (1986).
Handoll, H. D. thesis Univ. Oxford (1985).
Artymiuk, P. J., & Blake, C. C. F. J. molec. Biol. 152, 737–762. (1981).
McKenzie, H. A., & Shaw, D. C. Biochem. Int. 10, 23–31 (1985).
Schaer, J.-J., Milos, M., & Cox, J. A. FEBS Lett. 190, 77–80. (1985).
Koga, K. & Berliner, L. J. Biochemistry 24, 7257–7262(1985).
Chothia, C. & Lesk, A. M. Trends biochem. Sci. 111, 116–118 (1985).
Weaver, L. H. et al. J. molec. Evol., 21, 97–111 (1985).
Tufty, R. M. & Kretsinger, R. H. Science, 187, 167–169 (1975).
Bernstein, F. C., et al. J. molec. Biol. 112, 535–542, (1977).
Stuart, D. I. thesis, Bristol Univ. (1979).
Rossmann, M. G. & Argos, P. J. molec. Biol. 109, 99–129, (1977).
Blundell, T. L. & Johnson, L. N. in Protein Crystallography 147, (Academic, New York, 1976).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stuart, D., Acharya, K., Walker, N. et al. α-Lactalbumin possesses a novel calcium binding loop. Nature 324, 84–87 (1986). https://doi.org/10.1038/324084a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/324084a0
- Springer Nature Limited
This article is cited by
-
Are Physicochemical Properties Shaping the Allergenic Potency of Animal Allergens?
Clinical Reviews in Allergy & Immunology (2022)
-
Dissecting protein loops with a statistical scalpel suggests a functional implication of some structural motifs
BMC Bioinformatics (2011)
-
Mining protein loops using a structural alphabet and statistical exceptionality
BMC Bioinformatics (2010)
-
Analysis of local structure of Arg10 domain in apo-α-lactalbumin with a polarity-sensitive arginine-specific fluorescent probe
Science in China Series B: Chemistry (2009)
-
MSDmotif: exploring protein sites and motifs
BMC Bioinformatics (2008)