Abstract
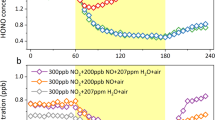
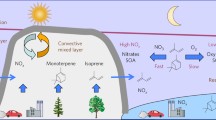
Nitrogen oxides (NOx) have a central role in the chemistry of the atmosphere, especially in key processes relating to ozone, hydroxyl-radical (OH) and acid formation1–7. High reactivity of NOx (lifetime of 0.5–2 days) precludes hemispheric-scale transport and it has been proposed that non-methane hydrocarbons present in the troposphere can transform NOx into its organic forms principally as peroxyacetyl nitrate (PAN)8,9. PAN is highly stable in the colder regions of the middle and upper troposphere and can provide a mechanism for NOx storage and transport. Once transported, PAN and its homologues can easily release free NOx in warmer atmospheric conditions. PAN is probably ubiquitous and its concentrations could exceed those of NOx in clean tropospheric conditions10–12. Here we present the first view of the global distribution of PAN based on extensive shipboard and aircraft measurements. PAN is more abundant in the Northern than in the Southern Hemisphere and in the continental than in the marine troposphere. In contrast to its behaviour in polluted atmospheres, PAN mixing ratios in winter greatly exceed those in summer. These measurements provide a basis for assessing the significance of PAN as a reservoir of NOx and for extending and validating reactive nitrogen chemistry theory in the troposphere.
Similar content being viewed by others
References
Haagen-Smit, A. J. Ind. Engng Chem. 44, 1342–1346 (1952).
Chamiedes, W. L. Geophys. Res. Lett. 5, 17–20 (1978).
Crutzen, P. J. A Rev. Earth planet. Sci. 7, 443–472 (1979).
Liu, S. C., Kley, D., McFarland, M., Mahlman, J. D. & Levy, H. J. geophys. Res. 85, 7546–7555 (1980).
Logan, J. A., McElroy, M. B., Wofsy, S. C. & Prather, M. J. J. geophys. Res. 96, 7210–7254 (1981).
Logan, J. A. J. geophys. Res. 85, 10785–10807 (1983).
Stedman, D. H. & McEwan, M. J. Geophys. Res. Lett. 10, 168–171 (1983).
Singh, H. B. & Hanst, P. L. Geophys. Res. Lett. 8, 941–944 (1981).
Aikin, A. C., Herman, J. R., Maier, E. J. & McQuillan, C. J. Planet Space Sci. 31, 1075–1082 (1983).
Singh, H. B. & Salas, L. J. Nature 302, 326–328 (1983).
Spicer, W. C., Holdren, M. W. & Keighley, G. W. Atmos. Envir. 17, 1055–1058 (1983).
Singh, H. B. et al. Nature 318, 347–349 (1985).
Singh, H. B. & Salas, L. J. Atmos. Envir. 17, 1507–1516 (1983).
Singh, H. B. & Salas, L. J. Geophys. Res. Lett. 9, 842–845 (1982).
MacFarland, M., Kley, D., Drummond, J. W., Schmeltekopf, A. L. & Winkler, R. H. Geophys. Res. Lett. 6, 605–608 (1979).
Ridley, Caroll, M. & Gregory, J. J. geophys. Res. (submitted).
Lee, Y. N. Pap. BNL-34735 presented at Conf. Gas-Liquid Chemistry of National Waters, Brookhaven natn. Lab., Upton, N.Y., 1–6 April (1984).
Kley, D., Drummond, J. W., McFarland, M. & Liu, S. C. J. geophys. Res. 86, 3153–3161 (1981).
Environmental Protection Agency, Air Quality Criteria for Ozone and Other Hydrocarbons, 600/8-78-004 (1979).
Kasting, J. F. & Singh, H. B. J. geophys. Res. (submitted).
Levine, J. S., Rinsland, E. P. & Tennille, G. M. Nature 318, 254–257 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Singh, H., Salas, L. & Viezee, W. Global distribution of peroxyacetyl nitrate. Nature 321, 588–591 (1986). https://doi.org/10.1038/321588a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/321588a0
- Springer Nature Limited
This article is cited by
-
A study of peroxyacetyl nitrate at a rural site in Beijing based on continuous observations from 2015 to 2019 and the WRF-Chem model
Frontiers of Environmental Science & Engineering (2020)
-
Wintertime characteristic of peroxyacetyl nitrate in the Chengyu district of southwestern China
Environmental Science and Pollution Research (2018)
-
Peroxyacetyl nitrate measurements by thermal dissociation–chemical ionization mass spectrometry in an urban environment: performance and characterizations
Frontiers of Environmental Science & Engineering (2017)
-
Ab initio study of the ground state and conformational stability of peroxyacetyl nitrate in internal rotation about the peroxide bond
Russian Chemical Bulletin (1998)
-
Measurements of peroxyacetylnitrate in the marine boundary layer over the Atlantic
Journal of Atmospheric Chemistry (1992)