Abstract
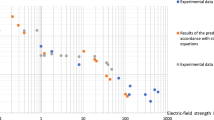
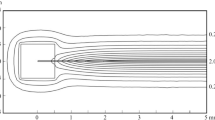
Negative ions are found in flames1 and are presumed to arise from electrons attaching to some unidentified flame species, possibly molecular oxygen2,3. Free electrons and positive ions are produced in a flame either by chemi-ionisation4 in CH + O → CHO+ + e− or by collisional ionisation5 of a metal, such as potassium, in K + M → K+ + e− + M, where M represents a third body. An atmospheric pressure flame is an interesting medium in which to measure the rate of appearance of negative ions; normally no more than one particle in 106 is electrically charged, there are usually no electric or magnetic fields and the attaching electrons are ‘thermalised’, that is, their energies are governed by the flame temperature. In addition, electron attachment is at much higher temperatures and pressures than normal6,7 and in a system free from wall effects. We present here a study of the kinetics of electron attachment to oxygen and water in flames. The rate coefficients suggest a surprisingly large negative temperature coefficient.
Similar content being viewed by others
References
Gaydon, A. G. & Wolfhard, H. G. Flames, their Structure, Radiation and Temperature, 3rd edn (Chapman and Hall, London, 1970).
Goodings, J. M., Bohme, D. K. & Ng, Chun-Wai Combust. Flame (in the press).
Hayhurst, A. N. & Kittelsen, D. B. Combust. Flame 31, 37 (1978).
Green, J. A. & Sugden, T. M. 9th. Int. Symp. Combust. 607 (1963).
Ashton, A. F. & Hayhurst, A. N. Combust. Flame 21, 69 (1973).
Pack, J. L. & Phelps, A. V. J. chem. Phys. 44, 1870 (1966); 45, 4316 (1966).
Phelps, A. V. Can. J. Chem. 47, 1783 (1969).
Burdett, N. A. & Hayhurst, A. N. Proc. R. Soc. A 355, 377 (1977).
Goodings, J. M., Hayhurst, A. N. & Ogden, J. E. (in preparation).
Bulewicz, E. M., James, C. G. & Sugden, T. M. Proc. R. Soc. A 235, 89 (1956).
Hayhurst, A. N., Mitchell, F. R. G. & Telford, N. R. Int. J. Mass Spec. Ion Phys. 7, 177 (1971).
Hayhurst, A. N. & Telford, N. R. Combust. Flame 28, 67 (1977).
Hayhurst, A. N. & Telford, N. R. Proc. R. Soc. A 322, 483 (1971).
Jensen, D. E. & Jones, G. A. Combust. Flame 32, 1 (1978).
Fehsenfeld, F. C., Ferguson, E. E. & Schmeltekopf, A. L. J. chem. Phys. 45, 1844 (1966).
Hershey, H. C., Zakin, J. L. & Simha, R. Ind. Eng. Chem. (Fund). 6, 413 (1967).
Ferguson, E. E., Fehsenfeld, F. C. & Schmeltekopf, A. L. Adv. Chem. Ser. 80, 83 (1969).
Hayhurst, A. N. & Kittelsen, D. B. Combust. Flame 28, 137 (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Goodings, J., Hayhurst, A. Kinetics of electron attachment to oxygen and water in flames. Nature 281, 204–206 (1979). https://doi.org/10.1038/281204a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/281204a0
- Springer Nature Limited