Abstract
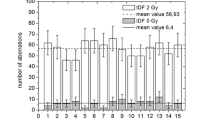
THE routine method for detecting genetic damage induced by environmental agents in man in vivo is to examine mitotic cells in short-term peripheral blood lymphocyte cultures for evidence of structural chromosome changes1,2. Chromosome radiosensitivity in lymphocytes irradiated in vivo and in vitro is similar3, and so dose–response relationships have been established for different qualities and quantities of ionising radiations after in vitro exposure of blood samples4. These curves are used to estimate doses received in cases of accidental overexposure to radiation1,2,5 and in prediction of the sensitivity of human germ cells to heritable radiation damage6. Their shape is used to predict the effect of dose rate on chromosome aberration frequencies7,8 and to elucidate the mechanisms of induction of such aberrations8. However, accurate dose–response curves can be obtained only if cells are examined for chromosome damage at the first post-irradiation mitosis, because aberrations decline with successive divisions9. Crossen and Morgan10 used the harlequin-staining technique10,12 on phytohaemagglutinin (PHA)-stimulated lymphocytes exposed to bromodeoxyuridine (BUdR) to distinguish cells at first and later mitoses in vitro (Fig. 1). They have shown that at the conventional sampling time, about 48 h after stimulation, up to 53% of cells may be in second mitosis. Another problem is that the cells examined in first division at 48 h may not be representative of the entire cell population. Several workers13–15 have claimed that lymphocytes with different rates of response to PHA stimulation, undergoing first mitosis at different times after stimulation, carry different frequencies of aberrations. They have interpreted this as a sign that lymphocytes in the peripheral blood constitute a heterogeneous population in terms of chromosome radiosensitivity. If this were true, little significance could be attached to the shape of the dose–response curves. We report here that we have used the harlequin technique to show (1) that in spite of radiation-induced mitotic delay16 high frequencies of cells in second mitosis may still be found at 48 h, and (2) that there is no difference in aberration frequency in first-division cells sampled at various intervals after irradiation. Correct dose–response curves can therefore now be obtained with this method and any sampling time can be used provided that observations are confined to non-harlequin-stained (that is, first division) cells.
Similar content being viewed by others
References
Dolphin, G. W. & Lloyd, D. C. J. med. Genet. 11, 181–189 (1974).
Bauchinger, M. in Mutagen Induced Chromosome Damage in Man (eds Evans, H. J. & Lloyd, D. L.) 9–13 (Edinburgh University Press, 1978).
Clemenger, J. F. & Scott, D. Int. J. Radiat. Biol. 24, 487–496 (1973).
Report of the United National Scientific Committee on the Effects of Atomic Radiation, 24th Session, Suppl. 13, A/7613 (General Assembly Official Records, New York, 1969).
Bender, M. A. Adv. Radiat. Biol. 3, 215–275 (1969).
Brewen, J. G. & Preston, R. J. in Radiation Research (eds Nygaard, O. F., Adler, H. I. & Sinclair, W. K.) 926–936 (Academic, New York, 1975).
Lloyd, D. C. et al. Int. J. Radiat. Biol. 28, 75–90 (1975).
Evans, H. J. in Human Radiation Cytogenetics (eds Evans, H. J., Court Brown, W. M. & McLean, A. S.) 20–36 (North-Holland, Amsterdam, 1967).
Heddle, J. A., Evans, H. J. & Scott, D. in Human Radiation Cytogenetics (eds Evans, H. J.,Court Brown, W. M. & McLean, A. S.) 6–19 (North-Holland, Amsterdam, 1967).
Crossen, P. E. & Morgan, W. F. Expl Cell Res. 104, 453–457 (1977).
Korenberg, J. R. & Freedlender, E. F. Chromosoma 48, 355–360 (1974).
Perry, P. & Wolff, S. Nature 251, 156–158 (1974).
Bender, M. A. & Brewen, J. G. Mutat. Res. 8, 383–399 (1969).
Beek, B. & Obe, G. Hum. Genet. 35, 57–70 (1976).
Steffen, J. A. & Michalowski, A. Mutat. Res. 17, 367–376 (1973).
Vulpis, N., Purrott, R. J. & Lloyd, D. C. Mutat. Res. 51, 145–148 (1978).
Lambert, B. et al. Hereditas 83, 163–174 (1976).
Conger, A. D. Radiat. Bot. 5, 81–96 (1965).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SCOTT, D., LYONS, C. Homogeneous sensitivity of human peripheral blood lymphocytes to radiation-induced chromosome damage. Nature 278, 756–758 (1979). https://doi.org/10.1038/278756a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/278756a0
- Springer Nature Limited
This article is cited by
-
Influence of mitotic delay on the results of biological dosimetry for high doses of ionizing radiation
Radiation and Environmental Biophysics (2005)
-
The production of chromosome structural changes by radiation
Experientia (1989)
-
Reduced frequency of baseline sister chromatid exchanges in lymphocytes grown in antibiotics and serum-excluded culture medium
Human Genetics (1983)
-
The effect of blood storage on differential chromosome staining of human lymphocytes
Experientia (1983)