Abstract
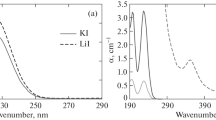
THE assumption of solute-solvent compound formation in certain iodine solutions is largely1, though not entirely2, based on spectroscopic evidence, including the violet shift of the visible absorption bond in associated solvents3, and the appearance of a very strong ultra-violet absorption band at 2970 A. in solutions of iodine in benzene4. There are several reasons why the spectroscopic evidence should be interpreted with caution. Solutions of bromine behave spectroscopically in a manner very similar to those of iodine, in that the visible absorption band is displaced to the violet in associated solvents, and in the appearance of a very strong ultra-violet band in the region 2500–2975 A. in several solvents5. It has never been stated just why solvation should cause these spectroscopic effects. On the other hand, Bayliss and Rees6 have shown that the violet shift of the visible absorption band of both iodine and bromine in associated solvents can be explained in terms of the physical effect of the hydrogen-bonded 'cage' of solvent molecules around each solute molecule, and Rees7 has obtained good quantitative agreement between the calculated and experimental displacements when water is the solvent.
Similar content being viewed by others
References
Kleinberg and Davidson, Chem. Rev., 42, 601 (1948).
Fairbrother, Nature, 160, 87 (1947).
Walker, Trans. Farad. Soc., 31, 1432 (1935).
Benesi and Hildebrand, J. Amer. Chem. Soc., 70, 2832 (1948).
Aitkin, Bayliss and Rees, Proc. Roy. Soc., A, 169, 234 (1938).
Bayliss and Rees, J. Chem. Phys., 8, 377 (1940).
Rees, J. Chem. Phys., 8, 429 (1940).
Bayliss, Cole and Green ; presented to Perth Meeting of Australian and New Zealand Assoc. Adv. Sci., August 1947.
Green and Rees (unpublished, private communication).
Mulliken and Rieke, Rep. Progr. Physics, 8, 231 (1941).
Bayliss ; presented to Perth Meeting of Australian and New Zealand Assoc. Adv. Sci., August 1947, in preparation for publication.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BAYLISS, N. Spectra of Iodine and Bromine Solutions. Nature 163, 764–765 (1949). https://doi.org/10.1038/163764a0
Issue Date:
DOI: https://doi.org/10.1038/163764a0
- Springer Nature Limited
This article is cited by
-
Chemical effects induced by gamma-irradiated alkali halides in organic emulsions
Journal of Radioanalytical and Nuclear Chemistry Articles (1990)
-
Interaction of Iodine with Aromatic Hydrocarbons
Nature (1949)