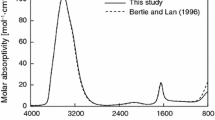
Abstract
A new analysis methodology of the ν 2 + ν 3 band of H2O developed by us earlier was applied for aqueous solutions of KCl, KBr, CsCl and CsBr. We isolate the effect of inter-molecular interactions among solute ions and solvent molecules on the near infrared spectra as the excess absorptivity, ε E, and then take its mole fraction derivative, \( \varepsilon_{\text{S}}^{\text{E}} \) (S stands for the solute). \( \varepsilon_{\text{S}}^{\text{E}} \) then signifies the effect of solute S on ε E, the transition moment of the ν 2 + ν 3 combination band due only to the intermolecular interactions. Together with the earlier similar studies on aqueous NaCl, NaBr, NaI, (CH3)4Cl, and (C2H5)4Cl, we found that ε E has apparently three universal bands among all the aqueous salts studied so far; a negative band centered at 4873 cm−1, a positive one at 5123 cm−1, and a negative band at 5263 cm−1. They were attributed to “solid-like”, “liquid-like” and “gas-like” H2O chromophores, respectively, depending on the local and instantaneous completeness of hydrogen bonding. Our recent thermodynamic studies indicated that K+, Cs+, and Cl− ions are “hydration centers” that are hydrated by a number of H2O molecules, but the bulk H2O away from hydration shells is left unperturbed. Br− is a “hydrophile” ion that forms hydrogen bonds directly to the fleetingly and yet permanently existing hydrogen bond network of H2O, but pins down the fluctuations inherent in pure H2O. The distinction between Cl− and Br− are apparent only in the behavior of \( \varepsilon_{\text{S}}^{\text{E}} \) at the 5123 cm−1 “liquid-like” chromophore band. The quantitative differences in the hydration numbers among Na+, K+, and Cs+ are not conspicuous in either of these three chromophores. We stress, however, that at the ε E level there seem to be three bands at 4873, 5123, and 5263 cm−1 that could be universal among aqueous solutions of electrolyte solutes.





Similar content being viewed by others
References
Koga, Y., Sebe, F., Minami, T., Otake, K., Saitow, K., Nishikawa, K.: Spectrum of excess partial molar absorptivity. I. Near infrared spectroscopic study of squeous acetonitrile and acetone. J. Phys. Chem. B. 113, 11928–11935 (2009)
Sebe, F., Nishikawa, K., Koga, Y.: Spectrum of excess partial molar absorptivity. Part II: a near infrared spectroscopic study of aqueous Na-halides. Phys. Chem. Chem. Phys. 14, 4433–4439 (2012)
Koga, Y., Sebe, F., Nishikawa, K.: Effects of tetramethyl- and tetraethylammonium chloride on H2O: calorimetric and near-infrared spectroscopic study. J. Phys. Chem. B. 117, 877–883 (2013)
Li, Q., Wang, N., Zhou, Q., Sun, S., Yu, Z.: Excess infrared absorption spectroscopy and its applications in the studies of hydrogen bonds in alcohol-containing binary mixtures. Appl. Spectrosc. 62, 166–170 (2008)
Ikehata, A.: Concept of differential molar absorptivity. 3rd Symposium of the NIR Division, The Spectroscopy Society of Japan. January 21, Osaka, Japan (2008)
Koga, Y.: Partial molar quantity of an intensive mother function. J. Chem. Phys. 137, 124503 (2012)
Fornes, V., Cahussidon, J.: An interpretation of the evolution with temperature of the ν(2) + ν(3) combination band in water. J. Chem. Phys. 68, 4667–4671 (1978)
Koga, Y.: 1-Propanol probing methodology: two-dimensional characterization of the effect of solute on H2O. Phys. Chem. Chem. Phys. 15, 14548–14565 (2013)
Koga, Y.: Solution thermodynamics and its application to aqueous solutions: a differential approach, chap. V. Elsevier B.V, Amsterdam (2007)
Koga, Y.: Solution thermodynamics and its application to aqueous solutions: a differential approach, chap. VI. Elsevier B.V, Amsterdam (2007)
Koga, Y.: Mixing schemes in aqueous solutions of nonelectrolytes: a thermodynamic approach. J. Phys. Chem. 100, 5172–5181 (1996)
Koga, Y.: Mixing schemes in binary aqueous solutions of non-electrolytes. Netsu Sokutei. 30, 54–65 (2003)
Morita, T., Westh, P., Nishikawa, K., Koga, Y.: How much weaker are the effects of cations than those of anions? the effects of K+ and Cs+ on the molecular organization of liquid H2O. J. Phys. Chem. B. 118, 8744–8749 (2014)
Saitow, K., Kobayashi, K., Nishikawa, K.: How are hydrogen bonds perturbed in aqueous NaClO4 solutions depending on the concentration?: a near infrared study of water. J. Solution Chem. 33, 689–698 (2004)
The Chemical Society of Japan edition Chemistry Handbook. Maruzen, Tokyo (1983)
Buchner, R., Hefter, G.: Interactions and dynamics in electrolyte solutions by dielectric spectroscopy. Phys. Chem. Chem. Phys. 11, 8984–8999 (2009)
Parsons, M.T., Westh, P., Davies, J.V., Trandum, C., To, E.C.H., Chiang, W.M., Yee, E.G.M., Koga, Y.: A thermodynamic study of 1-propanol–glycerol–H2O at 25 °C: Effect of glycerol on molecular organization of H2O. J. Solution Chem. 30, 1007–1028 (2001)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
While the ε E (ave.) seems to point approximately to zero at \( x_{\text{S}} = 0 \) for the 4873 cm−1 (Figs. 4a and 5b) and for the 5123 cm−1 (Figs. 4b and 5b) bands, that for 5236 cm−1, Figs. 4c and 5c, tend to extrapolate to a relatively large positive value at \( x_{\text{S}} = 0 \). For the NaCl, NaBr and NaI cases studied earlier [2], the ε E (ave.) value at 5263 cm−1 showed an initial increase from ε E (ave.) = 0 at \( x_{\text{S}} = 0 \). Thus, the next derivative, \( \varepsilon_{\text{S}}^{\text{E}} \), indicates a sharp bend to a lower slope region that was attributed to H2O-separated ion pairing [16] in the region \( x_{\text{S}} > 0.032 \) for NaCl, \( x_{\text{S}} > 0.036 \) for NaBr, and \( x_{\text{S}} > 0.040 \) for NaI. Here for KCl, KBr, CsCl, and CsBr, however, there are little signs of initial increases from ε E (ave.) = 0 at \( x_{S} = 0 \). We thus leave this set at 5263 cm−1 out of further discussion. It may be probable, however, that H2O-mediated ion pairing may be occurring at much lower mole fractions just as the case for (CH3)4NCl and (C2H5)4NCl [3].
At 4873 cm−1, Figs. 4a and 5a, and at 5123 cm−1, Fig. 4b and 5b, not all of the of ε E (ave.) values seem to extrapolate to zero at \( x_{\text{S}} = 0 \). Judging from the behavior of the equivalent ε E (ave.) values for NaCl, NaBr, and NaI, which smoothly extrapolate to zero at infinite dilution [2], we assume for the present study that the ones in which ε E (ave.) data do not extrapolate to zero at \( x_{\text{S}} = 0 \) are due to a constant shift of background and/or the incident beam in the original A data. In order to examine the effect of salt on ε E (ave.) of each chromophore, we evaluate the excess partial molar absorptivity, \( \varepsilon_{\text{S}}^{\text{E}} \), by Eq. 3. With the above assumption that the baseline is shifted, i.e. the values of ε E (ave.) are shifted by a constant amount within a series of a salt, the resulting \( \varepsilon_{\text{S}}^{\text{E}} \) would be correct. We drew a smooth curve through the data points as indicated in Figs. 4a, b and 5a,b. We read the data off the curve drawn at the interval of δ\( x_{\text{S}} = 0.002 \), and approximate the quotient \( (\delta \varepsilon^{\text{E}} ({\text{ave.}})/\delta x_{\text{S}} \) as the partial derivative of the far right of Eq. 3 [17]. The results of \( \varepsilon_{\text{S}}^{\text{E}} \) are plotted in Fig. 6 for 4873 cm−1 and Fig. 7 for 5123 cm−1.
The 4873 cm−1 chromophore in the ν 2 + ν 3 combination band of H2O was attributed as an “ice-like” portion of H2O [1–3], following from the NIR spectrum of solid ice and in comparison with that of liquid H2O [7]. According to Fig. 6a, b, the effect of addition of salt on ε E (ave.) is approximately constant and independent of \( x_{\text{S}} \) within the mole fraction range studied. This was also the case for NaCl and NaBr [2]. Furthermore, the value of \( \varepsilon_{\text{S}}^{\text{E}} \) for S = NaCl was about −500 ± 60 cm2·mol−1, while −600 ± 60 cm2·mol−1 for KCl (Fig. 6a) and −400 ± 60 cm2·mol−1 for CsCl (Fig. 6b). From our previous 1P-probing studies, all the ions involved here, Cl− [8], Na+ [8], K+ [13], and Cs+ [13], were found to belong to “hydration centers” that are hydrated by 2.3 ± 0.6, 5.2 (standard), 4.6 ± 0.8 and 1.1 ± 0.5 molecules of H2O, respectively, and leave the bulk H2O away from hydration shells unperturbed. This observation could be closely related to our findings that this 4873 cm−1 chromophore comes from “ice-like” patches of bulk liquid H2O that are left unperturbed by the presence of ions belonging to a “hydration center” [8]. The Br– ion, on the other hand, belongs to “hydrophile” that forms hydrogen bonds directly to the fleetingly and yet permanently existing hydrogen bond network of bulk H2O, but pins down the degree of fluctuation inherent in liquid H2O [8]. Now, Fig. 6a, b indicate that the values of \( \varepsilon_{\text{S}}^{\text{E}} \) for KBr and CsBr are constant at about −600 ± 60 cm2·mol−1. The same value was also obtained for NaBr [2]. This finding seems to be consistent with our earlier finding that a hydrophilic solute has no effect on the “ice-like” chromophore [1, 3], just as was found for NaBr [2].
Figure 7 shows the \( \varepsilon_{\text{S}}^{\text{E}} \) results at 5123 cm−1 for S = KCl, KBr, CsCl and CsBr. This chromophore was attributed to the “liquid-like” portion of H2O [1–3], again judging from the NIR spectra for ice and liquid H2O [7]. We earlier observed that the values of \( \varepsilon_{\text{S}}^{\text{E}} \) for NaCl stayed constant at about 1000 cm2·mol−1 while those for NaBr and NaI decreased from about 3000 cm2·mol−1 and 4000 cm2·mol−1, respectively, as \( x_{\text{S}} \) increased [2]. The same trends together with the similar values of \( \varepsilon_{\text{S}}^{\text{E}} \) are shown in Figs. 7a, b. Thus, the qualitative difference in their effects of Cl− versus those of Br− and I−, i.e., “hydration center” versus “hydrophile”, manifests itself in this 5123 cm−1 chromophore. Here, with the cation fixed, the difference of the effect of Cl− and Br− is also apparent only at this band at 5123 cm−1 for the present investigation of the Br− salts of K+ and Cs+. Thus, from the \( x_{\text{S}} \)-dependence of \( \varepsilon_{\text{S}}^{\text{E}} \), we confirmed that the 5123 cm−1 chromophore is the target band to elucidate the difference between the ions belonging to “hydration center” and to “hydrophile”.
Rights and permissions
About this article
Cite this article
Sebe, F., Nishikawa, K. & Koga, Y. Excess Partial Molar Absorptivity of Aqueous Solutions of KCl, KBr, CsCl and CsBr: Are There Three Universal Chromophores in the Excess Molar Absorptivity of the ν 2 + ν 3 Band of H2O for Aqueous Salt Solutions?. J Solution Chem 44, 1833–1843 (2015). https://doi.org/10.1007/s10953-015-0376-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-015-0376-3