Abstract
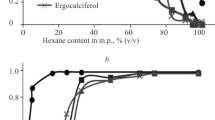
WHEN oils containing vitamins are submitted to molecular distillation1, the fractions removed as the temperature rises contain successively more vitamin until a maximum is reached, after which the potency falls rapidly to zero because all the vitamin has been eliminated. The elimination follows a simple law and the ideal elimination curve possesses a slightly skew shape which is easily recognizable. Deviations from the shape indicate imperfect conditions of distillation or the presence of more than one form of tne vitamin. The maximum of the curve can be located with an accuracy of ± 2° C., and is as definite a characteristic as a boiling point, if less precise. Separate elimination curves can be detected for more than one material if the maxima are 5° C. apart. When two potent materials yield different maxima, it is proof that they are different substances. Coincident maxima provide only partial evidence that the substances are identical. When oils containing traces of many impurities are distilled, their maxima occur in a fixed order, and if one can be identified, the others may be recognized. This enables dyes, etc., to serve as distillation pilots. For example, when traces of Celanthrene Red 3B and dimethyl-amino-anthraquinone are distilled with an oil containing the free vitamins A and D, the reddest fraction is found to hold the most vitamin A, and the bluest, the most vitamin D. The elimination curves of the two vitamins correspond almost exactly with those of the dyes (Fig. 1).
Similar content being viewed by others
References
C. R. Burch, Proc. Roy. Soc., A, 123, 271 (1929). E. W. Wash-burn, Bur. Stand. J. Research, 2, 476 (1929). F. E. Carr, Brit. Pat., 415, 088; Hickman, U.S. Pat. 1,995,559.
See A. L. Bacharach, NATURE, 138, 387 (Sept. 5, 1936).
C. E. Bills, ” Symposia on Quantitative Biology”, 3 (1935).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
HICKMAN, K. Identification of Vitamins by Molecular Distillation. Nature 138, 881–882 (1936). https://doi.org/10.1038/138881a0
Issue Date:
DOI: https://doi.org/10.1038/138881a0
- Springer Nature Limited