Abstract
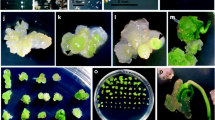
This study was conducted to compare characteristics of a wheat (Triticum aestivum L.) cell line to those of the maize (Zea mays L.) black Mexican sweet (BMS) cell line and to compare protoplasts isolated from suspension cells of these cell lines. The wheat cell line was established from immature-embryo derived callus of the experimental line ‘ND7532’ and was conditioned for growth in suspension culture. For both cell lines, measurements of packed cell volume (PCV), fresh weight (FW), and dry weight (DW) were taken at 3 day intervals from suspension cultures. Measurements of FW of calluses cultured from suspension cells of both cell lines were taken at 6 day intervals. The morphogenetic potential of the wheat ND7532 cell line was tested in both callus and suspension cultures using media promoting regeneration and/or organogenesis. Growth rates of ND7532 cells in suspension culture were comparable to those of BMS cells. However, relative growth rates of calluses recovered from ND7532 suspension cells were slower than those of calluses recovered from BMS suspension cells. The ND7532 cell line has very limited morphogenetic potential and has been maintained as rapidly growing callus tissue for 11 years. Yields of protoplasts from suspension cells of the two cell lines were comparable, though ND7532 protoplasts were typically smaller. The wheat cell line has is now designated ND7532-NM (nonmorphogenetic) and is available for cellular and molecular biology research.
Similar content being viewed by others
References
Ahmed KZ, Omirulleh S, Safi F & Dudits D (1997) Factors affecting transient expression of vector constructs in wheat protoplasts. Acta Biologica Hungarica 48: 209-220
Erdei L, Vigh L & Dudits D (1982) Isolation of a wheat (Triticum monococcum L.) cell line with altered membrane properties. Plant Physiol. 69: 572-574
Fellers JP, Guenzi AC & Taliaferro CM (1995) Factors affecting the establishment and maintenance of embryogenic callus and suspension cultures of wheat. Plant Cell Rep. 15: 232-237
Galbraith DW (1981) Microfluorimetric quantitation of cellulose biosynthesis by plant protoplasts using calcofluor white. Physiol. Plant. 53: 111-116
Hadlaczky G, Bisztray G, Pranznovsky T & Dudits D (1983) Mass isolation of plant chromosomes and nuclei. Planta 157: 278-285
Hahne G, Herth W & Hoffmann F (1983) Wall formation and cell division in fluorescence-labeled plant protoplasts. Protoplasma 115: 217-221
Kumpaisal R, Hashimoto T & Yamada Y (1988) Selection and characterization of S-2 aminoethyl-L-cysteine resistant wheat cell cultures. J. Plant Physiol. 133: 608-614
Larkin PJ (1976) Purification and viability determinations of plant protoplasts. Planta (Berl.) 128: 213-216
Mougin C, Polge N, Scalla R & Cabanne F (1991) Interactions of various agrochemicals with cytochrome P-450 dependent monoxoygenases of wheat cells. Pesticide Biochem. Physiol. 40: 1-11
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473-497
Redway FA, Vasil V, Lu D & Vasil IK (1990) Identification of callus types for long-term maintenance and regeneration from commercial cultivars of wheat (Triticum aestivum L.). Theor. Appl. Genet. 79: 609-617
Salas SL & Hille E (1974) Calculus: One and Several Variables (2nd edn). John Wiley & Sons, New York
SAS Institute (1985) SAS/STAT guide for personal computers, version 6. SAS Institute Inc., Cary, NC
Schaefer W & Sandermann Jr H (1988) Metabolism of pentachlorophenol in cell suspension cultures of wheat (Triticum aestivum L.). J. Agric. Food Chem. 36: 370-377
Schmidt B, Rivero C & Thiede B (1995) 3,4-Dichloroaniline N-glucosyl-and N-malonyltransferase activities in cell cultures and plants of soybean and wheat. Phytochemistry 39: 81-84
Sears RG & Deckard EL (1982) Tissue culture variability in wheat: callus induction and plant regeneration. Crop Sci. 22: 546-550
Sheridan WF (1982) Black Mexican sweet corn: its use for tissue cultures. In: Sheridan WF (ed) Maizefor Biological Research. (pp. 385-388). Plant Molec. Biol. Assoc.
Singer SR & McDaniel CN (1986) Analyzing growth in cell cultures. II. Effect of initial cell mass on growth. Can. J. Bot. 64: 238-341
Szabados L & Dudits D (1980) Fusion between interphase and mitotic plant protoplasts: induction of premature chromosome condensation. Exp. Cell Res. 127: 442-446
Szabados L, Hadlaczky G & Dudits D (1981) Uptake of isolated plant chromosomes by plant protoplasts. Planta 151: 141-145
Vasil V, Redway FA & Vasil IK (1990) Regeneration of plants from embryogenic suspension culture protoplasts of wheat (Triticum aestivum L.). Biotechnology 8: 429-434
Wang WC & Nguyen HT (1990) A novel approach for efficient plant regeneration from long-term culture of wheat. Plant Cell Rep. 8: 639-642
Weir B, Gu X, Wang M, Upadhyaya N, Elliot A & Brettell R (2001) Agrobacterium tumefaciens-mediated transformation of wheat using suspension cells as a model system and green fluorescent protein as a visual marker. Aust. J. Plant Physiol. 28: 807-818
Yakovleva GC & Dudits D (1993) Effects of an aminooxy analogue of putrescine on wheat cell cultures (Triticum monococcum L.). J. Plant Physiol. 142: 218-221
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Guenzi, A.C., Scheets, K., Green, J.L. et al. Development and Characterization of a Nonmorphogenetic Cell Line of Wheat (Triticum aestivum). Plant Cell, Tissue and Organ Culture 78, 23–28 (2004). https://doi.org/10.1023/B:TICU.0000020391.67996.44
Issue Date:
DOI: https://doi.org/10.1023/B:TICU.0000020391.67996.44