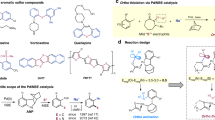
Abstract
A procedure was developed for monoarylation of sulfones with aryl halides in the presence of palladium complexes. Optimal reaction conditions were found, and the scope of application of the proposed procedure was determined. The arylation occurs only with those sulfones which are relatively strong CH acids; the corresponding monoarylated sulfones are formed in moderate to high yields. The arylation of carbanions derived from the sulfones and some other CH acids requires the presence of an additional equivalent of base. The presence of the latter is also necessary in stoichiometric reactions of carbanions with the palladium complex CF3C6H4Pd(PPh3)2Br; no reaction occurs in the absence of a base. A new mechanism of arylation was proposed, where the key stage is deprotonation of palladium intermediate ArPdL2CHXY which activates the reductive elimination stage.
Similar content being viewed by others
REFERENCES
Prilezhaeva, E.N., Poluchenie i svoistva organicheskikh soedinenii sery (Synthesis and Properties of Organic Sulfur Compounds), Belen'kii, L.I., Ed., Moscow: Khimiya, 1998, p. 115.
Najera, C. and Yus, M., Tetrahedron, 1999, vol. 55, p. 10546.
Houben-Weyl. Methoden der organische Chemie, Stuttgart: Georg Thieme, 1985, E11.
Kashin, A.N., Mitin, A.V., Beletskaya, I.P., and Wife, R., Tetrahedron Lett., 2002, vol. 43, p. 2539.
Culkin, D.A. and Hartwig, J.F., Acc. Chem. Res., 2003, vol. 36, p. 234.
Prim, D., Campagne, J.-M., Joseph, D., and Andriolet-ti, B., Tetrahedron, 2002, vol. 58, p. 2041.
Sakamoto, T., Katoh, E., Kondo, Y., and Yamanaka, H., Chem. Pharm. Bull., 1990, vol. 38, p. 1513.
Uno, M., Seto, K., and Takahashi, S., J. Chem. Soc., Chem. Commun., 1984, p. 932.
Bordwell, F.G. and Hughes, D.L., J. Org. Chem., 1980, vol. 45, p. 3314; Bordwell, F.G. and Hughes, D.L., J. Org. Chem., 1980, vol. 45, p. 3320.
Bordwell, F.G., Vanier, W.S., Matthews, W.S., Hendrick-son, J.B., and Skipper, P.L., J. Am. Chem. Soc., 1975, vol. 97, p. 7160.
Nelson, R.B. and Gribble, G.W., J. Org. Chem., 1974, vol. 39, p. 1425.
Ishii, Y., Ann. N.Y. Acad. Sci., 1972, vol. 239, p. 114; Ukai, T., Kawazura, H., and Ishii, Y., J. Organomet. Chem., 1974, vol. 65, p. 253.
Kohler, E.P. and Tishler, M., J. Am. Chem. Soc., 1935, vol. 57, p. 217.
Ashley, W.C. and Shriner, R.L., J. Am. Chem. Soc., 1932, vol. 54, p. 4410.
Hendrickson, J.B., Giga, A., and Wareing, J., J. Am. Chem. Soc., 1974, vol. 96, p. 2275.
Fitton, P. and Rick, E.A., J. Organomet. Chem., 1971, vol. 28, p. 279.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mitin, A.V., Kashin, A.N. & Beletskaya, I.P. Palladium-Catalyzed Arylation of Sulfones. Russian Journal of Organic Chemistry 40, 802–812 (2004). https://doi.org/10.1023/B:RUJO.0000044542.70121.04
Issue Date:
DOI: https://doi.org/10.1023/B:RUJO.0000044542.70121.04