Abstract
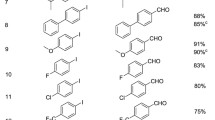
A new route to bidentate amide directing groups has been developed via the palladium(II)-catalyzed aminocarbonylation. Under atmospheric carbon monoxide pressure, using commercially available aryl iodides and aromatic amine derivatives as substrates, the three-component reaction proceeded smoothly to give the desired products in moderate-to-excellent yields with good functional-group compatibility.





Similar content being viewed by others
Notes
For selected reviews on transition metals catalyzed C–H functionalization.
References
Ma W, Kaplaneris N, Fang X, Gu L, Mei R, Ackermann L (2020) Org Chem Front 7:1022
Borpatra PJ, Deka B, Deb ML, Baruah PK (2019) Org Chem Front 6:3445
Dey A, Thrimurtulu N, Volla CMR (2019) Org Lett 21:3871
Rao W-H, Shi B-F (2016) Org Chem Front 3:1028
Chen Z, Wang B, Zhang J, Yu W, Liu Z, Zhang Y (2015) Org Chem Front 2:1107
Yang L, Huang H (2015) Chem Rev 115:3468
Gao K, Yoshikai N (2014) Acc Chem Res 47:1208
Wencel-Delord J, Droge T, Liu F, Glorius F (2011) Chem Soc Rev 40:4740
Giri R, Shi BF, Engle KM, Maugel N, Yu JQ (2009) Chem Soc Rev 38:3242
Guo X-K, Zhang L-B, Wei D, Niu J-L (2015) Chem Sci 6:7059
Zaitsev VG, Shabashov D, Daugulis O (2005) J Am Chem Soc 127:13154
Li Q, Huang J, Chen G, Wang S-B (2020) Org Biomol Chem 18:4802
Liu X, Zhang H, Yang F (2019) Bo Wang. Org Biomol Chem 17:7564
Ueno R, Natsui S, Chatani N (2018) Org Lett 20:1062
Kommagalla Y, Yamazaki K, Yamaguchi T, Chatani N (2018) Chem Commun 54:1359
Zhu L, Cao X, Li Y, Liu T, Wang X, Qiu R, Yin S-F (2017) Chin J Org Chem 37:1613
Vinayak B, NavyaSree P, Chandrasekharam M (2017) Org Biomol Chem 15:9200
Lin C, Chen Z, Liu Z, Zhang Y (2017) Org Lett 19:850
Iwasaki M, Miki N, Tsuchiya Y, Nakajima K, Nishihara Y (2017) Org Lett 19:1092
Sun S-Z, Shang M, Xu H, Cheng T-J, Li M-H, Dai H-X (2020) Chem Commun 56:1444
Hu F-P, Cui X-F, Lu G-Q, Huang G-S (2020) Org Biomol Chem 18:4376
Gao T-H, Wang C-M, Tang K-X, Xu Y-G, Sun L-P (2019) Eur J Org Chem 19:3005
Wan L, Qiao K, Yuan X, Zheng M-W, Fan B-B, Di ZC, Zhang D, Fang Z, Guo K (2017) Adv Synth Catal 359:2596
Liu J, Xue Z, Zeng Z, Chen Y, Chen G (2016) Adv Synth Catal 358:3694
Wang H-L, Shang M, Sun S-Z, Zhou Z-L, Laforteza BN, Dai H-X, Yu J-Q (2015) Org Lett 17:1228
Shang M, Sun S-Z, Dai H-X, Yu J-Q (2014) J Am Chem Soc 136:3354
Shang M, Wang H-L, Sun S-Z, Dai H-X, Yu J-Q (2014) J Am Chem Soc 136:11590
Ding Y, Han Y-Q, Wu L-S, Zhou T, Yao Q-J, Feng Y-L, Li Y, Kong K-X, Shi B-F (2020) Angew Chem Int Ed. https://doi.org/10.1002/anie.202004504
Zhou T, Jiang M-X, Yang X, Yue Q, Han Y-Q, Ding Y, Shi B-F (2020) Chin J Chem 38:242
Han Y-Q, Ding Y, Zhou T, Yan S-Y, Song H, Shi B-F (2019) J Am Chem Soc 141:4558
Yan S-Y, Han Y-Q, Yao Q-J, Nie X-L, Liu L, Shi B-F (2018) Angew Chem Int Ed 57:9093
Li Y, Liu Y-J, Shi B-F (2017) Adv Synth Catal 359:4117
Chen F-J, Zhao S, Hu F, Chen K, Zhang Q, Zhang S-Q, Shi B-F (2013) Chem Sci 4:4187
Fan C-L, Zhang L-B, Liu J, Hao X-Q, Niu J-L, Song M-P (2019) Org Chem Front 6:2215
Du C, Li P-X, Zhu X, Suo J-F, Niu J-L, Song M-P (2016) Angew Chem Int Ed 55:13571
Zhang L-B, Hao X-Q, Liu Z-J, Zheng X-X, Zhang S-K, Niu J-L, Song M-P (2015) Angew Chem Int Ed 54:10012
Hao X-Q, Chen L-J, Ren B, Li L-Y, Yang X-Y, Gong J-F, Niu J-L, Song M-P (2014) Org Lett 16:1104
Shen Y, Cindy Lee W-C, Gutierrez DA, Li JJ (2017) J Org Chem 82:11620
Mane RS, Bhanage BM (2017) Adv Synth Catal 359:2621
Gergely M, Boros B, Kollar L (2017) Tetrahedron 73:6736
Liu S, Deng Q, Fang W, Gong J-F, Song M-P, Xu M, Tu T (2014) Org Chem Front 1:1261
Wang T, Wang R, Wang W, Zhang A, Liu L (2018) J Organomet Chem 858:62
Mukherjee A, Subramanyam U, Puranik VG, Mohandas TP, Sarkar A (2005) Eur J Inorg Chem 1254
Korshin EE, Sabirova LA, Levin YA (2012) Synthesis 44:3512
Ouyang K, Xi Z (2013) Acta Chim Sin 71:13
Acknowledgements
We are grateful to the Key Science Research of Education Committee in Henan Province (19A150035), the Key Scientific and Technological Project of Henan Province (192102110222), the Program for Science & Technology Innovation Talents in Universities of Henan Province (14HASTIT016) and the Program of Science and Technology Innovation Talents of Henan Province (No. 2018JQ0011).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, T., Wang, X. et al. Palladium-catalyzed aminocarbonylation of aryl iodides with amines: efficient access to bidentate amide directing groups. Transit Met Chem 46, 29–35 (2021). https://doi.org/10.1007/s11243-020-00418-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-020-00418-4