Abstract
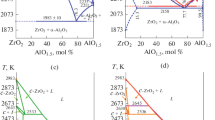
Isothermal sections at 1250 and 1650°C have been constructed for the Al2O3—ZrO2—Nd2O3 phase diagram, and the phase equilibria at those temperatures have been identified. No ternary compounds are found, and nor are there appreciable solid-solution ranges based on the components or binary compounds. Partially quasibinary sections have been observed: NdAlO3—(66.7 mole% ZrO2—33.3 mole% Nd2O3), AL—F, and β—F, which triangulate the ternary system, and the mechanism for the penetration of the phase Nd2Zr2O7 into the ternary system has been established.
Similar content being viewed by others
REFERENCES
I. A. Davtyan, V. B. Glushkova, and E. K. Keller, “A study of the ZrO2-Nd2O3 system: Regions rich in zirconium dioxide, ” Izv. AN SSSR, Neorgan. Materialy, 1, No. 5, 743–750 (1965).
Yoshio Sakka, Tohru S. Suzuki, Koji Morita, et al., “Colloidal processing and superplastic properties of finegrained zirconia-based ceramics, ” Key Engineering Materials, 206–213, 645–648 (2002).
E. Gregorova, J. Havrda, and W. Pabst, “ATZ ceramics prepared by slip-casting and centrifugal slip casting, ” Key Engineering Materials, 206–213, 369–372 (2002).
A. Rodriquez-Pulido, I. M. Ross, and W. M. Rainforth, “Processing and structural characterisation of 3Y-TZP ceramics resistant to hydrothermal ageing, ” Key Engineering Materials, 206–213, 1053–1056 (2002).
S. N. Lakiza, L. M. Lopato, and A. V. Shevchenko, “Interaction in the Al2O3-ZrO2-Y2O3 system, ” Poroshk. Metall., Nos. 9–10, 46–51 (1994).
F. Bertaut and F. Forrat, “Etude des combinaisons des oxydes des terres rares avec l'alumine et la galline, ” C. R. Acad. Sci., 243, No. 17, 1219–1222 (1956).
S. J. Schneider, R. S. Roth, and J. L. Waring, “Solid state reactions involving oxides of trivalent cations, ” J. Res. Nat. Bur. Stand., 65A, No. 4, 345–374 (1961).
N. A. Toropov and T. P. Kiseleva, “The alumina-neodymium oxide binary system and some data on the neodymium oxide-alumina-silica system, ” Zhurn. Neorgan. Khimii, 6, No. 10, 2353–2358 (1961).
N. A. Godina and E. K. Keller, “Formation conditions for the aluminates of lanthanum, praseodymium, and neodymium, ” Izv. AN SSSR, Ser. Khim., No. 1, 24–31 (1966).
D. Goldberg, “Contribution a l'etude des systems formes par l'alumine avec quelques oxydes de metaux trivalents et tetravalents, en particulier, l'oxyde de titane, ” Rev. Int. Hautes Temp. Refract., 5, No. 3, 181–194 (1968).
M. Mizuno, T. Yamada, and T. Nogushi, “Phase diagram of the system Al2O3-Nd2O3 at high temperatures, ” J. Ceram. Soc. Jap., 85, No. 2, 90–95 (1977).
E. Antic-Fidanchev and P. Caro, “Optical absorption spectrum of the β-alumina neodyme phase, ” C. R. Hebd. Seances Acad. Sci., Ser. C, 284, No. 13, 471–474 (1977).
J. P. Coutures, “The Al2O3-Nd2O3 phase diagram, ” J. Amer. Ceram. Soc., 68, No. 3, 103–107 (1986).
R. S. Roth, “Pyrochlore-type compounds containing double oxides of trivalent and tetravalent ions, ” J. Res. Nat. Bur. St., 56, No. 1, 17–25 (1956).
Y. Peres and M. Jorba, “ZrO2-rare earth oxides systems, ” Ann. Chim., 7, No. 7–8, 479–511 (1962).
A. Rouanet, “Contribution a l'etude des systemes zircone-oxydes des lanthanides au voisinage de la fusion, ” Rev. Int. Hautes Temp. Refract., 8, No. 2, 161–180 (1977).
E. I. Zoz, E. N. Fomichev, A. A. Kalashnik, et al., “Structure and properties of REE zirconates and hafnates, ” Zhurn. Neorgan. Khimii, 27, No. 1, 95–99 (1982).
V. B. Glushkova, V. K. Krzhizhanovskaya, V. I. Strakhov, et al., “Phase formation in the ZrO2-Nd2O3(Y2O3)-Al2O3(Cr2O3) systems and properties of materials based on them, ” Ogneupory i Tekhnicheskaya Keramika, No. 2, 16–21 (2001).
S. M. Zakiza and L. M. Lopato, “Interactions in the Al2O3-ZrO2-La2O3 system at 1250 and 1650°C, ” Poroshk. Metall., Nos. 7–8, 99–103 (2000).
S. M. Lakiza and L. M. Lopato, “Stable and metastable phase relations in the system Alumina-Zirconia-Yttria, ” J. Amer. Ceram. Soc., 80, No. 4, 893–902 (1997).
L. M. Lopato, A. V. Shevchenko, and A. E. Kushchevskii, “Research on highly refractory oxide systems, ” Poroshk. Metall., No. 1, 88–92 (1972).
V. P. Red'ko, The Physicochemical Study of the Compounds MnZr(Hf) 3 O 12 in the ZrO 2 (HfO 2 )-REE Oxide Systems, PhD Thesis [in Russian], Kiev (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lakiza, S.M., Red'ko, V.P. & Lopato, L.M. Phase Diagram of the Al2O3—ZrO2—Nd2O3 System. Part 1. Isothermal Sections of the Phase Diagram at 1250 and 1650°C. Powder Metallurgy and Metal Ceramics 42, 394–402 (2003). https://doi.org/10.1023/B:PMMC.0000008177.59188.3a
Issue Date:
DOI: https://doi.org/10.1023/B:PMMC.0000008177.59188.3a