Abstract
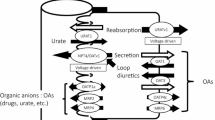
Purpose. Creatinine is excreted into urine by tubular secretion in addition to glomerular filtration. The purpose of this study was to clarify molecular mechanisms underlying the tubular secretion of creatinine in the human kidney.
Methods. Transport of [14C]creatinine by human organic ion transporters (SLC22A) was assessed by HEK293 cells expressing hOCT1, hOCT2, hOCT2-A, hOAT1, and hOAT3.
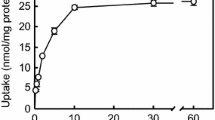
Results. Among the organic ion transporters examined, only hOCT2 stimulated creatinine uptake when expressed in HEK293 cells. Creatinine uptake by hOCT2 was dependent on the membrane potential. The Michaelis constant (Km) for creatinine transport by hOCT2 was 4.0 mM, suggesting low affinity. Various cationic drugs including cimetidine and trimethoprim, but not anionic drugs, markedly inhibited creatinine uptake by hOCT2.
Conclusion. These results suggest that hOCT2, but not hOCT1, is responsible for the basolateral membrane transport of creatinine in the human kidney.
Similar content being viewed by others
References
J. B. Pritchard and D. S. Miller. Mechanisms mediating renal secretion of organic anions and cations. Physiol. Rev. 73:765-796 (1993).
K. J. Ullrich. Specificity of transporters for ‘organic anions’ and ‘organic cations’ in the kidney. Biochim. Biophys. Acta 1197:45-62 (1994).
H. Koepsell. Organic cation transporters in intestine, kidney, liver, and brain. Annu. Rev. Physiol. 60:243-266 (1998).
K. Inui and M. Okuda. Cellular and molecular mechanisms of renal tubular secretion of organic anions and cations. Clin. Exp. Nephrol. 2:100-108 (1998).
K. Inui, S. Masuda, and H. Saito. Cellular and molecular aspects of drug transport in the kidney. Kidney Int. 58:944-958 (2000).
M. Okuda, H. Saito, Y. Urakami, M. Takano, and K. Inui. cDNA cloning and functional expression of a novel rat kidney organic cation transporter, OCT2. Biochem. Biophys. Res. Commun. 224:500-507 (1996).
D. Gründemann, V. Gorboulev, S. Gambaryan, M. Veyhl, and H. Koepsell. Drug excretion mediated by a new prototype of polyspecific transporter. Nature 372:549-552 (1994).
A. E. Busch, S. Quester, J. C. Ulzheimer, V. Gorboulev, A. Akhoundova, S. Waldegger, F. Lang, and H. Koepsell. Monoamine neurotransmitter transport mediated by the polyspecific cation transporter rOCT1. FEBS Lett. 395:153-156 (1996).
A. E. Busch, S. Quester, J. C. Ulzheimer, S. Waldegger, V. Gorboulev, P. Arndt, F. Lang, and H. Koepsell. Electrogenic properties and substrate specificity of the polyspecific rat cation transporter rOCT1. J. Biol. Chem. 271:32599-32604 (1996).
M. Okuda, Y. Urakami, H. Saito, and K. Inui. Molecular mechanisms of organic cation transport in OCT2-expressing Xenopus oocytes. Biochim. Biophys. Acta 1417:224-231 (1999).
Y. Urakami, M. Okuda, S. Masuda, H. Saito, and K. Inui. Functional characteristics and membrane localization of rat multispecific organic cation transporters, OCT1 and OCT2, mediating tubular secretion of cationic drugs. J. Pharmacol. Exp. Ther. 287:800-805 (1998).
D. Gründemann, G. Liebich, N. Kiefer, S. Koster, and E. Schömig. Selective substrates for non-neuronal monoamine transporters. Mol. Pharmacol. 56:1-10 (1999).
Y. Urakami, M. Okuda, S. Masuda, M. Akazawa, H. Saito, and K. Inui. Distinct characteristics of organic cation transporters, OCT1 and OCT2, in the basolateral membrane of renal tubules. Pharm. Res. 18:1528-1534 (2001).
U. Karbach, J. Kricke, F. Meyer-Wentrup, V. Gorboulev, C. Volk, D. Loffing-Cueni, B. Kaissling, S. Bachmann, and H. Koepsell. Localization of organic cation transporters OCT1 and OCT2 in rat kidney. Am. J. Physiol. 279:F679-F687 (2000).
M. Sugawara-Yokoo, Y. Urakami, H. Koyama, K. Fujikura, S. Masuda, H. Saito, T. Naruse, K. Inui, and K. Takata. Differential localization of organic cation transporters rOCT1 and rOCT2 in the basolateral membrane of rat kidney proximal tubules. Histochem. Cell Biol. 114:175-180 (2000).
Y. Urakami, M. Akazawa, H. Saito, M. Okuda, and K. Inui. cDNA cloning, functional characterization, and tissue distribution of an alternatively spliced variant of organic cation transporter hOCT2 predominantly expressed in the human kidney. J. Am. Soc. Nephrol. 13:1703-1710 (2002).
H. Motohashi, Y. Sakurai, H. Saito, S. Masuda, Y. Urakami, M. Goto, A. Fukatsu, O. Ogawa, and K. Inui. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J. Am. Soc. Nephrol. 13:866-874 (2002).
V. Gorboulev, J. C. Ulzheimer, A. Akhoundova, I. Ulzheimer-Teuber, U. Karbach, S. Quester, C. Baumann, F. Lang, A. E. Busch, and H. Koepsell. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 16:871-881 (1997).
L. Zhang, M. J. Dresser, A. T. Gray, S. C. Yost, S. Terashita, and K. M. Giacomini. Cloning and functional expression of a human liver organic cation transporter. Mol. Pharmacol. 51:913-921 (1997).
M. M. Bradford. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254 (1976).
J. Shannon. The renal excretion of creatinine in man. J. Clin. Invest. 14:403-410 (1935).
B. F. Miller and A. W. Winkler. The renal excretion of endogenous creatinine in man. Comparison with exogenous creatinine and inulin. J. Clin. Invest. 17:31-40 (1938).
G. M. Berlyne, H. Varley, S. Nilwarangkur, and M. Hoerni. Endogenous creatinine clearance and glomerular filtration rate. Lancet 2:874-876 (1964).
B. Hood, P. O. Attman, J. Ahlmen, and R. Jagenburg. Renal hemodynamics and limitations of creatinine clearance in determining filtration rate in glomerular disease. Scand. J. Urol. Nephrol. 5:154-161 (1971).
B. J. Carrie, H. V. Golbetz, A. S. Michaels, and B. D. Myers. Creatinine: an inadequate filtration marker in glomerular diseases. Am. J. Med. 69:177-182 (1980).
J. H. Bauer, C. S. Brooks, and R. N. Burch. Clinical appraisal of creatinine clearance as a measurement of glomerular filtration rate. Am. J. Kidney Dis. 2:337-346 (1982).
O. Shemesh, H. Golbetz, J. P. Kriss, and B. D. Myers. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 28:830-838 (1985).
F. Berglund, J. Killander, and R. Pompeius. Effect of trimethoprim-sulfamethoxazole on the renal excretion of creatinine in man. J. Urol. 114:802-808 (1975).
E. Burgess, A. Blair, K. Krichman, and R. E. Cutler. Inhibition of renal creatinine secretion by cimetidine in humans. Ren. Physiol. 5:27-30 (1982).
B. A. C. van Acker, G. C. M. Koomen, M. G. Koopman, D. R. de Waart, and L. Arisz. Creatinine clearance during cimetidine administration for measurement of glomerular filtration rate. Lancet 340:1326-1329 (1992).
B. Crawford. Depression of the exogenous creatinine/inulin or thiosulfate clearance ratios in man by diodrast and p-amino-hippuric acid. J. Clin. Invest. 27:171-175 (1948).
H. C. Burry and P. A. Dieppe. Apparent reduction of endogenous creatinine clearance by salicylate treatment. BMJ 2:16-17 (1976).
W. M. Barendt and S. H. Wright. The human organic cation transporter (hOCT2) recognizes the degree of substrate ionization. J. Biol. Chem. 277:22491-22496 (2002).
J. G. van den Berg, M. G. Koopman, and L. Arisz. Ranitidine has no influence on tubular creatinine secretion. Nephron 74:705-708 (1996).
J. H. Lin. Pharmacokinetic and pharmacodynamic properties of histamine H2-receptor antagonists. Relationship between intrinsic potency and effective plasma concentrations. Clin. Pharmacokinet. 20:218-236 (1991).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Urakami, Y., Kimura, N., Okuda, M. et al. Creatinine Transport by Basolateral Organic Cation Transporter hOCT2 in the Human Kidney. Pharm Res 21, 976–981 (2004). https://doi.org/10.1023/B:PHAM.0000029286.45788.ad
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000029286.45788.ad