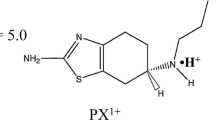
Abstract
Purpose. The aim of this study was to characterize the influence of pH and NaCl concentration on the transdermal iontophoretic transport of the dopamine receptor agonist rotigotine across human stratum corneum (HSC).
Methods. Rotigotine transport was studied in vitro in side by side diffusion cells according to the following protocol: 6 h of passive diffusion, 9 h of iontophoresis, and 5 h of passive diffusion. A current density of 0.5 mA cm−2 was used. The influence of donor phase pH (4, 5, and 6) and different concentrations of NaCl (0.07 and 0.14 M) on rotigotine iontophoretic flux were examined. The acceptor phase was phosphate-buffered saline (PBS) at pH 7.4 except in one series of experiments aimed to study the effects of rotigotine solubility on its iontophoretic transport. In this study, PBS at pH 6.2 was used. In separate studies, 14C-mannitol was used as a marker to determine the role of electro-osmosis during iontophoresis.
Results. The estimated iontophoretic steady-state flux (Fluxss) of rotigotine was influenced by the pH of the donor solution. At a drug donor concentration of 0.5 mg ml−1, the iontophoretic flux was 30.0 ± 4.2 nmol cm−2 h−1 at pH 6 vs. 22.7 ± 5.5 nmol cm−2 h−1 at pH 5. However, when the donor concentration was increased to 1.4 mg ml−1, no significant difference in iontophoretic rotigotine transport was observed between pH 5 and 6. Increase of NaCl concentration from 0.07 M to 0.14 M resulted in a decrease of the rotigotine Fluxss from 22.7 ± 5.5 nmol cm−2 h−1 to 14.1 ± 4.9 nmol cm−2 h−1. The contribution of electro-osmosis was estimated less than 17%. Probably due to the lipophilic character of the drug, impeding the partitioning of rotigotine from HSC to the acceptor compartment, steady-state transport was not achieved during 9 h of iontophoresis.
Conclusions. Both pH and NaCl concentration of the donor phase are crucial on the iontophoretic transport of rotigotine. Electro-repulsion is the main mechanism of the iontophoretic transport of rotigotine.
Similar content being viewed by others
references
A. Lledo. Dopamine agonists: the treatment for Parkinson's disease in the XXI century? Parkinsonism Relat. Disord. 7:51–58 (2000).
J. Van der Weide, J. B. de Vries, P. G. Tepper, D. N. Krause, M. L. Dubocovich, and A. S. Horn. N-0437: a selective D-2 dopamine receptor agonist in in vitro and in vivo models. Eur. J. Pharmacol. 147:249–258 (1988).
V. P. Calabrese, K. A. Lloyd, P. Brancazio, E. Cefali, P. Martin, J. J. Wall, and D. Sica. N-0923, a novel soluble dopamine D2 agonist in the treatment of parkinsonism. Mov. Disord. 13:768–774 (1998).
T. K. Gerding, B. F. Drenth, H. J. Roosenstein, R. A. de Zeeuw, P. G. Tepper, and A. S. Horn. The metabolic fate of the dopamine agonist 2-(N-propyl-N-2-thienylethylamino)-5-hydroxy-tetralin in rats after intravenous and oral administration. I. Disposition and metabolic profiling. Xenobiotica 20:515–524 (1990).
T. K. Gerding, B. F. Drenth, R. A. de Zeeuw, P. G. Tepper, and A. S. Horn. The metabolic fate of the dopamine agonist 2-(N-propyl-N-2-thienylethylamino)-5-hydroxytetralin in rats after intravenous and oral administration. II. Isolation and identification of metabolites. Xenobiotica 20:525–536 (1990).
P. J. Swart and R. A. de Zeeuw. Extensive gastrointestinal metabolic conversion limits the oral bioavailability of the dopamine D2 agonist N-0923 in freely moving rats. Pharmazie 47:613–615 (1992).
P. J. Swart, W. E. Oelen, A. P. Bruins, P. G. Tepper, and R. A. de Zeeuw. Determination of the dopamine D2 agonist N-0923 and its major metabolites in perfused rat livers by HPLC-UV-atmospheric pressure ionization mass spectrometry. J. Anal. Toxicol. 18:71–77 (1994).
D. R. Friend. Transdermal delivery of contraceptives. Crit. Rev. Ther. Drug Carrier Syst. 7:149–186 (1990).
J. T. Hutton, L. V. Metman, T. N. Chase, J. L. Juncos, W. C. Koller, R. Pahwa, P. A. LeWitt, A. Samii, J. K. Tsui, D. B. Calne, C. H. Waters, V. P. Calabrese, J. P. Bennett, R. Barrett, and J. L. Morris. Transdermal dopaminergic D(2) receptor agonist therapy in Parkinson's disease with N-0923 TDS: a double-blind, placebo-controlled study. Mov. Disord. 16:459–463 (2001).
L. V. Metman, M. Gillespie, C. Farmer, F. Bibbiani, S. Konitsiotis, M. Morris, H. Shill, W. Bara-Jimenez, M. M. Mouradian, and T. N. Chase. Continuous transdermal dopaminergic stimulation in advanced Parkinson's disease. Clin. Neuropharmacol. 24:163–169 (2001).
P. J. Swart, W. L. Weide, and R. A. de Zeeuw. In vitro penetration of the dopamine D2 agonist N-0923 with and without Azone. Int. J. Pharm. 87:67–72 (1992).
B. H. Sage. Iontophoresis. In E. W. Smith and H. I. Maibach (eds.), Percutaneous Penetration Enhancer, CRC Press Inc., Boca Raton, 1995, pp. 351–368.
A. Luzardo-Alvarez, M. B. Delgado-Charro, and J. Blanco-Mendez. Iontophoretic delivery of ropinirole hydrochloride: effect of current density and vehicle formulation. Pharm. Res. 18:1714–1720 (2001).
R. Van der Geest, M. Danhof, and H. E. Bodde. Iontophoretic delivery of apomorphine. I: In vitro optimization and validation. Pharm. Res. 14:1798–1803 (1997).
G. L. Li, M. Danhof, and J. A. Bouwstra. Effect of elastic liquid-state vesicle on apomorphine iontophoresis transport through human skin in vitro. Pharm. Res. 18:1627–1630 (2001).
G. L. Li, M. Danhof, and J. A. Bouwstra. Iontophoretic delivery of apomorphine in vitro: physicochemic considerations. Pharm. Res. 18:1509–1513 (2001).
G. L. Li, R. Van der Geest, L. Chanet, E. van Zanten, M. Danhof, and J. A. Bouwstra. In vitro iontophoresis of R-apomorphine across human stratum corneum. Structure-transport relationship of penetration enhancement. J. Control. Rel. 84:49–57 (2002).
G. L. Li, M. Danhof, P. M. Frederik, and J. A. Bouwstra. Pretreatment with a water-based surfactant formulation affects transdermal iontophoretic delivery of R-apomorphine in vitro. Pharm. Res. 20:653–659 (2003).
R. Van der Geest, T. Van Laar, J. M. Gubbens-Stibbe, H. E. Bodde, and M. Danhof. Iontophoretic delivery of apomorphine. II: an in vivo study in patients with Parkinson's disease. Pharm. Res. 14:1804–1810 (1997).
G. L. Li. Transdermal Iontophoretic Delivery of R-Apomorphine for the Treatment of Patients with Parkinson's Disease, Ph.D. Thesis, Leiden University, Leiden, The Netherlands, 2003.
R. Van der Geest, M. Danhof, and H. E. Bodde. Validation and testing of a new iontophoretic continuous flow through transport cell. J. Control. Rel. 51:85–91 (1998).
N. Kanikkannan, J. Singh, and P. Ramarao. In vitro transdermal iontophoretic transport of timolol maleate: effect of age and species. J. Control. Rel. 71:99–105 (2001).
S. Y. Oh, S. Y. Jeong, T. G. Park, and J. H. Lee. Enhanced transdermal delivery of AZT (Zidovudine) using iontophoresis and penetration enhancer. J. Control. Rel. 51:161–168 (1998).
Anonymous. N-0923 Hydrochloride General Information and Properties, internal report, Schwarz Pharma, 2001.
L. Wearley and Y. W. Chien. Enhancement of the in vitro skin permeability of azidothymidine (AZT) via iontophoresis and chemical enhancer. Pharm. Res. 7:34–40 (1990).
L. Wearley, L. Jue-Chen, and Y. W. Chien. Iontophoresis-facilitated transdermal delivery of verapamil I. In vitro evaluation and mechanistic studies. J. Control. Rel. 8:237–250 (1989).
D. Marro. R. H. Guy and, M. B. Delgado-Charro. Characterization of the iontophoretic permselectivity properties of human and pig skin. J. Controlled Release 70:213–217 (2001).
J. Hirvonen and R. H. Guy. Iontophoretic delivery across the skin: electro-osmosis and its modulation by drug substances. Pharm. Res. 14:1258–1263 (1997).
D. Marro, Y. N. Kalia, M. B. Delgado-Charro, and R. H. Guy. Contributions of electromigration and electro-osmosis to iontophoretic drug delivery. Pharm. Res. 18:1701–1708 (2001).
M. J. Pikal. The role of electroosmotic flow in transdermal iontophoresis. Adv. Drug Deliv. Rev. 46:281–305 (2001).
A. Jadoul, L. M. Dunbar, D. Ellis, and V. Preat. Modification induced on stratum corneum structure after in vitro iontophoresis: ATR-FTIR and X-ray scattering studies. J. Control. Rel. 42:165–173 (1996).
A. Jadoul, J. A. Bouwstra, and V. Preat. Effects of iontophoresis and electroporation on the stratum corneum. Review of the biophysical studies. Adv. Drug Deliv. Rev. 35:89–105 (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nugroho, A.K., Li, G.L., Danhof, M. et al. Transdermal Iontophoresis of Rotigotine Across Human Stratum Corneum in Vitro: Influence of pH and NaCl Concentration. Pharm Res 21, 844–850 (2004). https://doi.org/10.1023/B:PHAM.0000026438.57787.10
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000026438.57787.10