Abstract
This research study involves the development of an olanzapine (OLZ) formulation using various chemical penetration enhancers (CPEs) for transdermal delivery. The aim of this study was to obtain the initial data needed about the effects of various CPEs on the skin permeation of OLZ. The effects of the selected CPEs were examined by studying the permeation profiles of OLZ from formulations applied to human cadaver skin samples. A control formulation of OLZ in propylene glycol (PG) was prepared and compared against formulations containing chemical penetration enhancers. Five different CPEs (oleic acid (OA), cineole (Cin), isopropyl alcohol (IPA), Tween 80 (T80), and N-methyl pyrrolidone (NMP)) at 5% w/w were individually added to the formulation containing OLZ in PG. The in vitro permeation study was carried out using vertical Franz diffusion cells mounted with human cadaver skin. Samples from the receptor compartment of the cell were collected at 2 h, 4 h, 8 h, 12 h, and 24 h at room temperature. The amount (µg/cm2) of permeated drug (OLZ) was measured using a validated HPLC method, and the percentage (%) of OLZ permeated was calculated. Based on the data obtained, different CPEs were found to have a significant impact on OLZ permeability compared to the control formulation. The most effective chemical penetration enhancer was shown to be 5% w/w OA with a 3.3-fold increase in enhancement ratio (ER). The rank of order for the highest concentration of OLZ permeated from each of CPE containing formulation was as follows: OA > Cin > IPA > T80 > NMP. The most effective chemical penetration enhancer was OA but the cytotoxic study using human fibroblast cells suggests that OA may not be safe due to its cytotoxic effects.
Similar content being viewed by others
Introduction
The skin is considered one of the largest organs in the human body, which continuously serves as a self-regulating armor against the loss of water from the body (Trommer and Neubert 2006). It also prevents pollutants and other chemicals from entry into the body, regulates body temperature, and provides a fairly robust barrier to drug permeation (Vig et al. 2017). However, the skin is an attractive route for the transdermal delivery of drugs due to its extensive surface area. Many substances, including sunscreens, antiseptic agents, and insect-repellent formulations, are designed to stay on the skin’s surface (topical application), while others such as nitroglycerin and nicotine are intended to pass through the skin layers (transdermal application) in order to reach sites inside the body or underneath the skin (Supe and Takudage 2021). In other words, transdermal drug delivery can be defined as the concept by which a medication dissipates through the different skin layers and into the circulatory system of the body to produce a therapeutic effect (Garnier and Croft 2002).
Comparing the transdermal delivery of medications to traditional pathways of administration, there are many benefits of transdermal delivery, including the absence of hepatic first-pass metabolism, decreased adverse reactions due to more stable blood level profiles, simple usage, and increased length of drug therapeutic activity (Abraham et al. 1995; Wortmann et al. 2010). However, the primary barrier component of the skin structure, which is the stratum corneum, acts as a strong defense against the permeation of a variety of substances, and this skin layer has to be altered in a non-damaging manner to make sure that drugs are transported through the skin in an adequate therapeutic amount (Kanikkannan et al. 2005).
Comprehending the physiology and chemistry of the skin is crucial for effectively utilizing the process of percutaneous drug absorption. The epidermis, hypodermis, and hypodermis (the subcutaneous layer) are the three primary histological layers that make up the skin (Benson 2005). The epidermal skin layer can be further divided into five main parts: the stratum germinativum, stratum spinosum, stratum granulosum, stratum lucidum, and stratum corneum are the layers present in the epidermis, which has a thickness of about 0.1 to 1.5 mm. The epidermis is the site of cell division, keratin and lipid production, and melanin production. The thickest layer of the epidermis is the squamous cell layer. It facilitates the movement of specific compounds into and out of the body. The stratum corneum (also known as the “horny layer”) is composed of around 10–30 thin layers of anucleated, keratinized, dead skin cells that form an organized protein, water, and lipid structure (Vardy et al. 2001; Benson 2005). The 1.5–4-mm-thick dermis is found directly below the epidermis. It has lymph and blood vessels, sweat glands, oil-producing sebaceous glands, hair follicles, collagen, and elastin. However, it is worth mentioning that drugs cannot effectively penetrate the stratum corneum, the topmost layer, which acts as a barrier within the skin (Dubey et al. 2012; Baishakhi et al. 2016). Therefore, the physicochemical characteristics of the drug have a significant impact on how much percutaneous drug absorption occurs when a topical or transdermal medication is applied. Only a small subset of medications can be administered via the transdermal pathway due to the dependence on physical and chemical characteristics of their molecules (Dubey et al. 2012; Haq et al. 2020). In transdermal drug delivery systems (TDDS), the effective penetration of drug molecule is crucial. Basically, there are several ways for drugs to penetrate the skin. Three different routes allow micro- and macromolecules to permeate the skin: (a) The lipid matrix, which is present in the spaces between the cells of keratinocytes, serves as the intercellular route and is mostly preferred by lipophilic molecules; (b) the intracellular (transcellular) route, which passes via the corneocytes; and (c) the trans-appendageal route passes through sweat and sebaceous glands and hair follicles (Cevc 1997; Desai et al. 2010). Following topical medicine usage, molecules interact with the skin’s surface where they come into contact with sebum, cellular debris, microbes, and additional substances that barely impact penetration (Moser et al. 2001). Drugs that take the intracellular route move through the stratum corneum’s cells explicitly, whereas those that take the intercellular pathway penetrate slowly around the cells (Malan et al. 2002; Desai et al. 2010).
The intracellular pathway is challenging to move because it must first enter each cell’s lipophilic membrane, then pass through the hydrophilic core of the cell, and finally exit the lipophilic membrane. Due to this fact, as per their polarity, both hydrophilic and lipophilic molecules prefer to pass through this route (Bolzinger et al. 2012). The intracellular pathway also needs some hydrophilic properties to pass through the corneocyte’s interior. However, the intercellular pathway has an additional penetrative polar pathway. The use of absorption boosters, or substances that react with skin components to increase medicinal flow, has long been a method of expanding the variety of medicines which are successfully administered through the dermal pathway (Dragicevic et al. 2015).
The usage of penetration enhancers could significantly increase the number of actives suitable for transdermal administration (Mathur et al. 2010). According to the lipid-protein partitioning (LPP) concept, permeation enhancers employ one of three main mechanisms to exert their effects: (i) disrupting the lipid matrix of the stratum corneum, (ii) interacting with intracellular proteins (Roy et al. 2017), or (iii) enhancing the ability of the medication or solvent to penetrate into the stratum corneum. The LPP concept helps identify substances that modify the components of the stratum corneum and the structural pathway for lipid diffusion. Permeation enhancers affect the desmosomes that maintain cohesion between corneocytes, leading to fissuring of the intercellular lipids and splitting of SC squames (Williams and Barry 2012). Permeation enhancer acts by enhancing the solubility of the drug in the formulation (Dragicevic et al. 2015). Kolliphor CS 20 was proven to increase penetration of econazole nitrate through the nail (Puri et al. 2022). The penetration enhancers were able to increase the solubility of the various drug molecules. As drug concentration increases, it leads to a high concentration gradient across the skin, thus increasing the permeation. Various formulations such as emulsion, solid dispersion, nanoemulsion, emulsomes, and ethosomes have been formulated to enhance solubility and bioavailability (Patel et al. 2016, Bahr et al. 2019, Parikh and Sawant 2019, Kapadia et al. 2021, Patel and Patel 2021, Ajjarapu et al. 2022, Shah et al. 2022, Modi et al. 2023, Patel et al. 2023).
As absorption enhancers, a wide range of chemically structured compounds have been studied. Fatty acids and alcohols, pyrrolidones, amides, surfactants, and urea and its derivatives are some of these substances. The variances in each class of enhancer’s framework and physicochemical characteristics are the key aspects that determine the extent of penetration enhancement (Williams and Barry 2012).
Olanzapine (OLZ) is an antipsychotic medication that is primarily used to treat symptoms of schizophrenia and bipolar disorder. It is available in an oral formulation. Oral administration of OLZ shows first-pass metabolism of the drug that reduced the availability of OLZ in systemic circulation by over 40% (Jawahar et al. 2018). The transdermal drug delivery of OLZ as an alternative route increases the bioavailability of the OLZ.
In the present study, we aimed to increase the low skin permeation potential of olanzapine by using various chemical penetration enhancers. This research has used five CPEs with various physicochemical characteristics to examine the efficacy and processes of olanzapine absorption across human cadaver skin. These CPEs include N-methyl-pyrrolidone (NMP), Tween 80 (T80), cineole (Cin), isopropyl alcohol (IPA), and oleic acid (OA).
Materials used in study
Chemicals
The drug substance OLZ investigated in the study was obtained from Thermo Fisher Scientific, MA, USA. Gattefosse Corporation, Paramus, NJ, USA, provided Transcutol® P (TRC) as a gift for research and development purposes. Cineole (Cin), HPLC grade acetonitrile and water, propylene glycol (PG), Tween 80 (T80), N-methyl-2-pyrrolidone (NMP), isopropyl alcohol (IPA), polyethylene glycol 400 (PEG 400), limonene, ethanol, and orthophosphoric acid were obtained from Sigma-Aldrich, Saint Louis, MO, USA. The phosphate-buffered saline (PBS) solution, which has a pH of 7.4, was created by dissolving one PBS tablet in 100 mL of water. PBS tablets were bought from MP Biomedicals in Solon, OH, USA. Dermatomed human cadaver skin from the posterior torso region of a 59-year-old male was purchased from New York Firefighter Skin Bank, New York, NY, USA. Note: the cadaver tissue sample was obtained from an accredited US Tissue Bank, and this is listed as exempt from the Institutional Review Board. Human dermal fibroblast (HDFa) cells were procured from the American Type Culture Collection (ATCC), VA, USA. Dulbecco’s Modified Eagle Medium (DMEM) and fetal bovine serum (FBS) were purchased from Thermo Fisher Scientific, MA, USA.
Cell culture
A human dermal fibroblast (HDFa) cells were cultured in DMEM supplemented with 10% FBS and 1% penicillin–streptomycin solution and incubated at 37 °C in an incubator with a humidified 5% carbon dioxide environment as per the ATCC guidelines. After reaching 80–90% confluency, the cells were subcultured. The HDFa cells were seeded onto 96-well plates at a density of 8 × 103 cells per well and incubated overnight at 37 °C in 5% CO2.
Methods
Solubility determination
OLZ solubility was investigated in different penetration enhancers using an excess amount of OLZ in the 5-mL vehicles. An excess amount of OLZ was added to PG, Cin, OA, IPA, NMP, ethanol, PEG400, PBS, limonene, and T80 followed by vortexing for 5 min and sonication in a water bath for 60 min at room temperature. The samples were mixed for 48 h at room temperature to increase the solubility of the drug substance into the solvents (Dugar et al. 2016, Rathod et al. 2020, Virani et al. 2023a). After 48 h, the mixtures were centrifuged at 10,000 rpm to separate dissolved (supernatant) and undissolved drug (sediment). Further, the supernatants were passed through 0.2-µm polypropylene syringe filter to remove the undissolved drug. The filtrate supernatants were diluted in the mobile phase and quantified by using the validated high-performance liquid chromatography (HPLC) method (described below).
HPLC method
HPLC was developed to determine the concentration of OLZ in the solubility and permeation samples. The HPLC equipment used was an Agilent 1100 series coupled with UV detection (with diode array detector (DAD)) and Agilent ChemStation software (OpenLab CDS, ChemStation Edition, Rev. C.01.10, Agilent Technologies, Santa Clara, CA, USA). The method was validated with respect to linearity, precision, accuracy, and repeatability for the drug molecule. For OLZ, a standard concentration range from 0.1 µg/mL to 100 µg/mL was utilized to generate a calibration curve with a coefficient of correlation, R2 > 0.99. Table 1 shows HPLC details for the quantification of OLZ.
In vitro skin permeation study of olanzapine
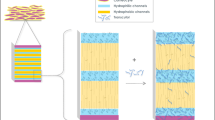
The dermatomed human cadaver skin, obtained from the posterior torso of a 59-year-old male donor, was stored at × 80 °C until use. The sample was obtained from the New York Firefighter Skin Bank, NY, USA. Prior to the study, the skin was thawed in pH 7.4 phosphate-buffered saline (PBS) at room temperature for 5 min. The skin is then cut into approximately 2 cm2 pieces (Matharoo et al. 2023) and hydrated in PBS pH 7.4 for 10 min before the permeation study. Each skin piece was mounted between the donor and receptor chambers of the Franz diffusion cells (Logan Instruments, Somerset, NJ, USA). The stratum corneum, the outermost layer of the skin, faced upward toward the donor chamber, while the lower part of the skin was in contact with the receptor chamber (Virani et al. 2023b). The diffusion area for permeation was 0.64 cm2. The receptor chamber was filled with 5.0 mL of PBS containing 30% v/v of PEG 400 at pH 7.4. A magnetic stirrer was used to continually stir the media in the receptor chamber at 600 rpm. The temperature was maintained at 32 °C ± 0.5 °C. The receptor chamber was allowed to equilibrate with the skin for 15 min before applying the dose. The donor phase, which contained 0.5 mL of a suspension of the compound of interest (OLZ) in PG, was applied to the stratum corneum in the donor chamber. The suspension was either alone or with the addition of 5% w/w of penetration enhancers. Samples of 300 µL were collected from the sampling arm of the Franz cells at specific time intervals, 2, 4, 8, 12, and 24 h. After each sampling, the collected samples were replenished with fresh receptor media which were kept at 32 °C. The cumulative amounts of drug were corrected for the sample concentrations removed from the cell receptors. All the samples collected at different time points were analyzed using a validated HPLC method described in the earlier section.
Formulation preparation
The control formulation OLZ in the PG without penetration enhancer was prepared by addition of the 1% w/w of OLZ in PG. The test formulations were OLZ in PG with penetration enhancers, and these were prepared by addition of the 1% w/w of OLZ in PG with 5% w/w of OA, Cin, T80, NMP, and IPA alone (Table 2).
Skin deposition quantification of OLZ
At the end of the permeation study, the skin samples were removed from Franz diffusion cells, cut around the diffusional area, and washed with ethanol to remove formulation residual. After this step, the skin samples were air-dried and accurately weighed to determine the initial weight. The dried skin samples were then placed into BeadBug tubes. The skin samples inside the tubes were cut into small pieces using scissors. To extract the drug from the skin samples, 1 mL of ethanol was added to each sample tube. These BeadBug tubes are designed for sample homogenization and extraction. The sample was homogenized using BeadBug™ Microtube homogenizer, D1030 (Benchmark Scientific, Sayreville, NJ, USA). Following that, the samples were sonicated to extract the drug from the skin. The sample tubes were centrifuged at 10,000 rpm for 10 min. Centrifugation assisted separation of the skin residue from the liquid extract. The liquid extract was then filtered through a 0.45-µm polypropylene filter (Virani et al. 2023a). The content of OLZ in the filtrate was analyzed using a validated HPLC method described in the earlier section.
Cytotoxicity assays
To determine in vitro cytotoxicity of olanzapine and the different excipients, HDFa were treated with 0- (control) to 250-μM concentrations of olanzapine solution in media only and different formulations diluted in media. Cell viability was determined 24 h later using the Alamar Blue assay (Invitrogen, CA, USA). Each drug was tested in triplicate experiments repeated at least three times). The fluorescence intensity from the samples was measured by using a microplate reader (Tecan, Männedorf, Switzerland).
Data analysis
Using the established HPLC method, the transdermal penetration of OLZ from various formulations over a 24-h period was recorded. The cumulative amount of OLZ that penetrated through a unit area of skin as a function of time was plotted to create a permeation profile. The slope of the linear component of the graph was used to determine the flux (J) value. The average OLZ flux was calculated by performing individual experiments five times. The enhancement ratio (ER) and flux (J) for each chemical penetration enhancer were calculated using the following equations:
The statistical analysis of the data was evaluated by using one-way ANOVA and Student’s t-test. The results were concluded statistically significant, if the p-value is less than or equal to 0.05. All calculations were evaluated using Statistical 12.5 software (StatSoft Inc., Tulsa, OK, USA).
Results and discussion
The development of a suitable and improved formulation using various chemical penetration enhancers to potentially deliver olanzapine (OLZ) drug through the skin is described in the current study. This includes a selection of appropriate solubilizing agents, various chemical penetration enhancers, and a study (in vitro permeation test) evaluating the impact of chemical penetration enhancers on the drug’s permeability through the human cadaver skin as compared to a control formulation.
Solubility determination of olanzapine in selected solvents
Preliminary solubility determination of OLZ was carried out using different solvents. OLZ is a highly lipophilic drug, and it belongs to the BCS Class II (low solubility, high permeability) category which exhibits poor aqueous solubility. The maximum solubility of the OLZ was observed in the solvent PEG 400 as 45.79 ± 1.62 mg/mL. The solubility results of OLZ (mg/mL) in different solubilizing agents are presented in Table 3.
The solubility of OLZ in PBS was observed as the lowest which was 1.28 ± 0.04 mg/mL. The solubility of OLZ in PBS can further be increased significantly by adding 20% PEG400 (5.71 ± 0.23 mg/mL) and 30% PEG400 (11.50 ± 1.67 mg/mL). To ensure the presence of sink conditions, PBS with the addition of 30% PEG400 v/v was used as receptor media during the permeation study. Results demonstrated that OLZ showed the highest solubility in the solvent PEG 400 (45.79 ± 1.62 mg/mL) alone compared to other vehicles. The solubility results of OLZ in PEG-400 are 2.2-fold higher than IPA (20.05 ± 0.40 mg/mL) and 1.8-fold higher than oleic acid (24.71 ± 1.10 mg/mL) and at 25 °C.
Solubility of OLZ in PG (2.29 ± 0.1 0 mg/mL) was considered further in this research study owning to the fact that PG is the most commonly used solvent and other vehicles (OA, Cin, IPA, T80, and NMP) can be used at different stages of this research.
Effect of chemical penetration enhancers
The barrier challenge offered by the skin’s outmost layer—the stratum corneum (SC) during the development of an optimized transdermal formulation with an appropriate penetration rate, can be overcome by the addition of various penetration enhancers to the formulation which can reversibly modify the barrier function of SC and make passage for the drug to travel through the skin into the epidermis/dermis layer (Chen et al. 2014, Chen et al. 2016, Parhi et al. 2020, Virani et al. 2023b). CPEs are widely used in dermal formulations to increase the solubility of lipophilic actives (Ezealisiji and Okorie 2018; Hmingthansanga et al. 2022). In this study, a total of five CPEs (oleic acid, cineole, isopropyl alcohol, Tween 80, and N-methyl pyrrolidone) were added individually in concentration of 5% w/w to the formulation containing drug (OLZ) in PG. The suitability of each chemical penetration enhancer and its mechanism of action and success rate are further explained in this manuscript.
To evaluate the effect of CPEs on OLZ skin permeation, various CPEs were incorporated at a fixed concentration of 5% (w/w) in a PG solution. Six formulations (one control formulation and five test formulations, each containing 5% w/w of different CPEs) were developed to study skin permeation effect offered by CPEs. The permeation profile of all the formulations was studied in vitro by IVPT. Skin permeation across human cadaver skin for six formulations in five replicates, for 24 h (receptor sample interval 0, 2, 4, 8, 12, 24 h), was evaluated, and the results are summarized in Table 4.
The table shows all CPEs used in the study have significantly increased the permeation rate (transdermal flux) of OLZ. It is clear from this table that out of five CPEs used in the current study, two of the CPEs, i.e., cineole and OA, have doubled (2.0-fold) and tripled (3.3-fold) ER compared to the control formulation, respectively. No significant difference between the ERs of NMP, T80 (1.7-fold), and IPA (1.8-fold) was observed.
Oleic acid
Oleic acid (OA) is a naturally occurring fatty acid found in various fats and oils. OA is a mono-saturated fatty acid and is a multifunctional pharmaceutical excipient which offers the properties of being an effective chemical penetration enhancer, antioxidant, and anti-inflammatory when used in topical formulations (Atef et al. 2022). OA as a chemical penetration enhancer has shown a significant increase in SC permeation of doxycycline microparticles containing OA as compared to the control formulation. The study indicated OA acts by disordering densely packaged intracellular lipid domains of SC and thus allowing the drug to penetrate the SC into the dermal layers (Aliyah et al. 2021). A research study of zaltoprofen transdermal delivery has investigated the use of OA as a penetration enhancer confirming OA’s dermal safety and acceptability in topical administration for not causing any skin irritation (Baek et al. 2013). A recent study of delivering adapalene topically also supports OA as an effective chemical penetration enhancer (Salimi et al. 2021). The optimized formula of ketoprofen gel containing 5% OA as a penetration enhancer exhibited improved anti-inflammatory activity compared to that available commercially (Sharma et al. 2020). OA disturbs the skin barrier function by disrupting SC lipid organization which is equivalent to increasing SC lipid temperature to 50 °C. Commercially available NSAID topical formulations contain OA as a penetration enhancer. A generic formulation of diclofenac 2% containing OA as a penetration enhancer and reference product containing diclofenac 2% containing oleyl alcohol as CPE are equivalent in in vitro penetration study determining and validating the method parameters and describing discriminatory power of the test method as well as in pivotal penetration study on industrial scale batches of generic product (Slíva 2021).
Cineole
One of the extensively used penetration enhancers is a monoterpene compound, i.e., cineole (Cin) which is the major component of eucalyptus oil and has been shown to increase permeation of lipophilic and hydrophilic drugs in human skin and rat skin. Cineole has shown promising results as a chemical penetration enhancer in transdermal delivery of Huperzine A through a microemulsion formulation (Shi et al. 2013; Moghimi et al. 2016). Apart from permeation enhancement property, cineole has shown a successful effect of inhibition of microorganisms such as bacteria, fungi, and parasites. This antimicrobial property of Cin is attributed to its capacity to interact with the biofilm of microorganism and extracting the essential elements of the cell and causing the ultimate cell death (Li et al. 2022). In a recent study, cineole in concentration of 5% was reported to increase the skin penetration enhancement ratio of dimethyl fumarate (DMF) to 5.3-fold (Ameen and Michniak-Kohn 2017).
Isopropyl alcohol
Isopropyl alcohol is a secondary short-chain alcohol also known as isopropanol and is widely used as a skin penetration enhancer in research as well as commercial dermal or transdermal products. It is used as a penetration enhancer in a commercial topical gel of diclofenac (trade name: Voltarol, Emugel) commercial transdermal patch of testosterone (trade name: Axiron and Tostran) along with other CPEs (Lane 2013). IPA has exhibited enhanced skin penetration and total skin absorption for the drug benzoic acid (Zhang et al. 2017). IPA was also used as a hydrophilic solvent and a penetration enhancer for the topical delivery of albumin. IPA caused an enhancement of albumin permeation (Shokri et al. 2014). IPA was used as a penetration enhancer for the aloe vera Emulgel (Mahuli and Anandamoy 2020).
Tween 80
Tween 80 is commercially used as a nonionic hydrophilic surfactant in the topical and transdermal formulations. Tween 80 is reported to extract intercellular lipids in SC and thus promote diffusion of the drugs and subsequent absorption of drugs. Tween 80 helps to achieve enhanced drug permeation through the skin by increasing the fluidity and solubility of the lipid contents of SC and by binding with keratin filaments that results in disruption within corneocyte (Kumar et al. 2015).
The synergistic effect of OA and Tween 80 for transdermal delivery of disulfiram further confirms a promising direction for the use of Tween 80 in topical formulations (Saitoh et al. 2023). In another study, Tween 80 has acted as an effective penetration enhancer for the transdermal delivery of diclofenac sodium through polymeric patches (Abdullah et al. 2023). T80 used in concentration of 1% w/w has shown increased ER and penetration for lorazepam as compared to the control sample containing water PG (50:50 v/v) (Nokhodchi et al. 2003).
N-Methyl pyrrolidone (NMP)
NMP belongs to the pyrrolidones group of penetration enhancers and is a widely studied enhancer amount in this group (Williams and Barry 2012). NMP is one of the most studied CPE which was first introduced in semisolid preparations in the early 1980s. The increased drug penetration through the skin in the presence of NMP is attributed to NMP’s ability to interact with keratins and lipids of the SC which then solubilize the lipid bilayer (Cilurzo et al. 2014).
A study assessing percutaneous permeation of tetrahydropalmatine (THP) Emulgels with various CPEs including NMP showed the highest cumulative permeation amount of THP in the presence of NMP used as a chemical penetration enhancer (Li et al. 2011, Javadzadeh et al. 2015), while recently another study showed a limited success of NMP as a penetration enhancer for captopril when formulated in a matrix type transdermal patch (Ganesh Sharma et al. 2012). NMP has multiplied the transdermal flux of ibuprofen 16-folds and flux of flurbiprofen by 3-folds from films (Saini et al. 2014, Roy et al. 2017).
Skin permeation studies
The effectiveness of the chemical penetration enhancers was determined by comparing permeation profile of OLZ with or without CPE. The cumulative amount of the permeated drug (OLZ) is plotted against time. The results of these permeation studies from all formulations are depicted in Figs. 1 and 2.
Longer time effect of each penetration enhancer can be seen from the drug permeation profile (Fig. 1). At the end of the 24-h study, the control formulation showed the lowest amount of OLZ permeated through the skin. The permeated amount of OLZ from the formulations containing 5% CPEs was significantly (p < 0.05) higher than the control formulation and exhibited the effectiveness of the penetration enhancers. The addition of 5% OA into the formulation has resulted in a significant increase (p < 0.05) in the amount of permeated drug (133.1 ± 19.1 µg/cm2) compared to using the control formulation with PG (40.60 ± 5.00 µg/cm2) after 24 h. As shown in Fig. 1, the permeated amount of OLZ from all formulations containing CPEs ranked as oleic acid > cineole > IPA > Tween 80 > NMP.
Our solubility study result indicated that OLZ has the lowest solubility in cineole as compared to NPM, T80, and IPA. However, cineole shows higher permeation than these studied permeation enhancers. In addition to solubilization, high permeation could be due to cineole possessing weaker cohesive forces, and hence, less energy is required for its molecules to H-bond with the skin ceramide polar heads which can increase the permeation (Jain et al. 2002). Our results of the skin deposition study (Fig. 3) also show that formulation with cineole has a higher amount of OLZ present in the skin layer as compared to NPM, T80, and IPA. Additionally, Ameen et al. demonstrated a comparable outcome in the skin permeation of dimethyl fumarate (DMF) by cineole (Ameen and Michniak-Kohn 2017). Cineole at a concentration of 5% V/V exhibits a DMF solubility of 13.9 ± 0.81 mg/mL, comparable to 13.8 ± 0.14 mg/mL at 5% V/V for Transcutol P. However, the flux rates are notably higher for cineole with a value of 108.5 ± 17.5 μg/cm2/h and 42.1 ± 4.81 μg/cm2/h with Transcutol P.
OA was the most effective penetration enhancer exhibiting the highest transdermal flux of OLZ, i.e., 5.6 ± 0.75 µg/cm2/h. OA significantly increased (p < 0.05) the flux of OLZ 3.3-fold, from 1.71 ± 0.2 to 5.6 ± 0.75 µg/cm2/h. The OLZ fluxes with cineole and IPA were 3.40 ± 0.41 µg/cm2/h and 3.13 ± 0.25 µg/cm2/h, respectively. The difference between the flux of NMP (2.83 ± 0.32 µg/cm2/h) and T80 (2.87 ± 0.25 µg/cm2/h) was not significant (p > 0.05).
Skin deposition quantification of OLZ
In Fig. 3, the amount of OLZ (measured in nanogram) detected after 24 h of permeation study through human cadaver skin (measured in mg) is presented. From the results, it is evident that the addition of 5% of OA as penetration enhancers significantly (p < 0.05) increases the amount of OLZ in the skin as compared to control formulation.
Cytotoxicity assays for the tested formulations
The formulation comprising F1, F2, F3, and F4 demonstrated significant potential in a delivery system for transdermal administration of olanzapine, along with suitable excipients. This formulation (Fig. 4) shows enhanced cell viability compared to olanzapine solution, particularly at concentrations of 170, 200, and 250 µM. F1 employed propylene glycol as the main solvent across all formulations, which, when combined with olanzapine, proved to be a promising and safe option for transdermal delivery.
Viability and proliferation of human dermal fibroblasts (HDFa), as measured by Alamar Blue assay, were observed with varying concentrations (0–250 μM) of olanzapine solution or in different solutions and surfactants (F1, F2, F3, F4, F5, and F6) after 24-h incubation (37 °C, 5% CO2) in DMEM medium supplemented with 10% FBS
F2 incorporates cineole, also known as eucalyptol, which is a natural organic compound found in essential oils of eucalyptus trees and other plants (Ince et al. 2018). Extensive research has explored its diverse biological activities, including anti-inflammatory and antioxidant properties. It is suggested that cineole protects the skin from damage and contributes to increased cell viability (Ho et al. 2020). However, further studies are required to fully comprehend the potential applications of cineole in skincare formulations, specifically regarding its effects on human dermal fibroblast cells.
F3 employs isopropyl alcohol (IPA), an antiseptic agent, which was found to improve cell viability compared to olanzapine alone. Furthermore, F4, the combination of N-methyl pyrrolidone with propylene glycol, exhibited a favorable combination as a carrier for olanzapine, ensuring safety in transdermal delivery (Jouyban et al. 2010). Conversely, the combination of propylene glycol with oleic acid (F5) significantly decreased cell viability. This may be attributed to oleic acid’s potential to induce oxidative damage in fibroblasts, as observed in a study by Romana-Souza et al. (Romana-Souza et al. 2020). Additionally, F6, the combination of propylene glycol with Tween 80, also led to reduced cell viability as reported by Hoda et al. that polysorbate 80 has minimal cytotoxic effects (Hoda et al. 2016). In summary, F1, F2, F3, and F4 all attenuated the killing effects observed with olanzapine solution. This observation suggests the potential use of these formulations in drug delivery systems for olanzapine.
Overall, the permeation of OLZ was significantly increased in the presence of the various permeation enhancers oleic acid, cineole, isopropyl alcohol, Tween 80, and N-methyl pyrrolidone. Furthermore, the use of these permeation enhancers in microemulsion formulation can create a synergistic effect that leads to an increment in drug permeation (Virani et al. 2023a, b).
Conclusions
This research study was designed with the aim to collect the initial data on the effect of various chemical penetration enhancers (CPEs) on the transdermal permeability of the antipsychotic drug olanzapine (OLZ). The results of in vitro human cadaver skin permeation studies from all the formulations with or without CPEs were compared. According to the results, when compared with the formulation without the penetration enhancer (control formulation), the drug permeated was increased in the presence of all the other five CPEs used at a concentration of 5% w/w. The results of the present study revealed that different CPEs produced a significant effect on the penetration of the OLZ drug through the skin. The highest permeated amount of OLZ was observed in the presence of oleic acid. However, after conducting a cytotoxicity study on the formulations, it was determined that while oleic acid showed enhanced permeation effects, it also exhibited detrimental effects on the skin. Consequently, it may not be a suitable choice as a permeation enhancer. In conclusion, transdermal delivery of olanzapine may be a promising approach to the oral route with an appropriately optimized formulation.
Abbreviations
- OLZ:
-
Olanzapine
- CPEs:
-
Chemical penetration enhancers
- PG:
-
Propylene glycol
- OA:
-
Oleic acid, cineole
- IPA:
-
Isopropyl alcohol
- Cin:
-
Cineole
- T80:
-
Tween 80
- NPM:
-
N-Methyl pyrrolidone
- TDDS:
-
Transdermal drug delivery systems
- LPP:
-
Lipid-protein partitioning
- PBS:
-
Phosphate-buffered saline
- HDFa:
-
Human dermal fibroblast
- ATCC:
-
American Type Culture Collection
- FBS:
-
Fetal bovine serum
- DAD:
-
Diode array detector
- SC:
-
Stratum corneum
- DMF:
-
Dimethyl fumarate
- THP:
-
Tetrahydropalmatine
References
Abdullah HM, Farooq M, Adnan S, Masood Z, Saeed MA, Aslam N, Ishaq W (2023) Development and evaluation of reservoir transdermal polymeric patches for controlled delivery of diclofenac sodium. Polym Bull 80(6):6793–6818
Abraham MH, Chadha HS, Mitchell RC (1995) The factors that influence skin penetration of solutes. J Pharm Pharmacol 47(1):8–16
Ajjarapu S, Banda S, Basim P, Dudhipala N (2022) Melt fusion techniques for solubility enhancement: a comparison of hot melt extrusion and KinetiSol® technologies. Sci Pharm 90(3):51
Aliyah A, Oktaviana WW, Dwipayanti KS, Erdiana AP, Utami RN, Permana AD (2021) Enhanced skin localization of doxycycline using microparticles and hydrogel: effect of oleic acid as penetration enhancer. Pharmaciana 11(239):10–12928
Ameen D, Michniak-Kohn B (2017) Transdermal delivery of dimethyl fumarate for Alzheimer’s disease: effect of penetration enhancers. Int J Pharm 529(1–2):465–473
Atef B, Ishak RA, Badawy SS, Osman R (2022) Exploring the potential of oleic acid in nanotechnology-mediated dermal drug delivery: an up-to-date review. J Drug Deliv Sci Technol 67:103032
Baek J-S, Lim J-H, Kang J-S, Shin S-C, Jung S-H, Cho C-W (2013) Enhanced transdermal drug delivery of zaltoprofen using a novel formulation. Int J Pharm 453(2):358–362
Bahr MN, Modi D, Patel S, Campbell G, Stockdale G (2019) Understanding the role of sodium lauryl sulfate on the biorelevant solubility of a combination of poorly water-soluble drugs using high throughput experimentation and mechanistic absorption modeling. J Pharm Pharm Sci 22:221–246
Baishakhi B, Ram A, Gowda D, Anjali S, Atul S, Osmani RAM (2016) Recent trends and advances in fungal drug delivery. J Chem Pharm Res 8(4):169–178
Benson HA (2005) Transdermal drug delivery: penetration enhancement techniques. Curr Drug Deliv 2(1):23–33
Bolzinger M-A, Briançon S, Pelletier J, Chevalier Y (2012) Penetration of drugs through skin, a complex rate-controlling membrane. Curr Opin Colloid Interface Sci 17(3):156–165
Cevc G (1997) Drug delivery across the skin. Expert Opin Investig Drugs 6(12):1887–1937
Chen Y, Quan P, Liu X, Wang M, Fang L (2014) Novel chemical permeation enhancers for transdermal drug delivery. Asian J Pharm Sci 9(2):51–64
Chen J, Jiang Q-D, Chai Y-P, Zhang H, Peng P, Yang X-X (2016) Natural terpenes as penetration enhancers for transdermal drug delivery. Molecules 21(12):1709
Cilurzo F, Vistoli G, Selmin F, Gennari CG, Musazzi UM, Franzé S, Lo Monte M, Minghetti P (2014) An insight into the skin penetration enhancement mechanism of N-methylpyrrolidone. Mol Pharm 11(3):1014–1021
Desai P, Patlolla RR, Singh M (2010) Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol Membr Biol 27(7):247–259
Dragicevic N, Atkinson JP, Maibach HI (2015) Chemical penetration enhancers: classification and mode of action. Percutaneous penetration enhancers chemical methods in penetration enhancement: modification of the stratum corneum. pp 11–27
Dubey A, Prabhu P, Kamath J (2012) Nano structured lipid carriers: a novel topical drug delivery system. Int J PharmTech Res 4(2):705–714
Dugar RP, Gajera BY, Dave RH (2016) Fusion method for solubility and dissolution rate enhancement of ibuprofen using block copolymer poloxamer 407. AAPS PharmSciTech 17:1428–1440
Ezealisiji KM, Okorie HN (2018) Size-dependent skin penetration of silver nanoparticles: effect of penetration enhancers. Appl Nanosci 8(8):2039–2046
Ganesh Sharma N, Sanadya J, Kaushik A, Dwivedi A (2012) Penetration enhancement of medicinal agents. Int Res J Pharm 3(5):2230–8407
Garnier T, Croft SL (2002) Topical treatment for cutaneous leishmaniasis. Curr Opin Investig Drugs 3:538–44
Haq A, Chandler M, Michniak-Kohn B (2020) Solubility-physicochemical-thermodynamic theory of penetration enhancer mechanism of action. Int J Pharm 575:118920
Hmingthansanga V, Singh N, Banerjee S, Manickam S, Velayutham R, Natesan S (2022) Improved topical drug delivery: role of permeation enhancers and advanced approaches. Pharmaceutics 14(12):2818
Ho C-L, Li L-H, Weng Y-C, Hua K-F, Ju T-C (2020) Eucalyptus essential oils inhibit the lipopolysaccharide-induced inflammatory response in RAW264. 7 macrophages through reducing MAPK and NF-κB pathways. BMC Complement Med Ther 20:1–11
Hoda M, Pajaniradje S, Shakya G, Mohankumar K, Rajagopalan R (2016) Anti-proliferative and apoptosis-triggering potential of disulfiram and disulfiram-loaded polysorbate 80-stabilized PLGA nanoparticles on hepatocellular carcinoma Hep3B cell line. Nanomedicine 12(6):1641–1650
Ince B, Dadaci M, Kilinc I, Oltulu P, Yarar S, Uyar M (2018) Effect of cineole, alpha-pinene, and camphor on survivability of skin flaps. Turkish J Med Sci 48(3):644–652
Jain AK, Thomas NS, Panchagnula R (2002) Transdermal drug delivery of imipramine hydrochloride.: I. Effect of terpenes. J Control Release 79(1–3):93–101
Javadzadeh Y, Adibkia K, Hamishekar H (2015) Transcutol®(diethylene glycol monoethyl ether): a potential penetration enhancer. Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Modification of the Stratum Corneum. pp 195–205
Jawahar N, Hingarh PK, Arun R, Selvaraj J, Anbarasan A, Sathianarayanan S, Nagaraju G (2018) Enhanced oral bioavailability of an antipsychotic drug through nanostructured lipid carriers. Int J Biol Macromol 110:269–275
Jouyban A, Fakhree MAA, Shayanfar A (2010) Review of pharmaceutical applications of N-methyl-2-pyrrolidone. J Pharm Pharm Sci 13(4):524–535
Kanikkannan N, Babu R, Singh M (2005) Structure–activity relationship of chemical penetration enhancers. Percutaneous penetration enhancers. CRC Press- Taylor & Francis group, New York, pp 31–48
Kapadia R, Parikh K, Jain M, Sawant K (2021) Topical instillation of triamcinolone a cetonide-loaded e mulsomes for posterior ocular delivery: statistical optimization and in vitro-in vivo studies. Drug Deliv Transl Res 11:984–999
Kumar P, Singh SK, Mishra DN, Girotra P (2015) Enhancement of ketorolac tromethamine permeability through rat skin using penetration enhancers: an ex-vivo study. Int J Pharm Investig 5(3):142
Lane ME (2013) Skin penetration enhancers. Int J Pharm 447(1–2):12–21
Li C, Liu C, Liu J, Fang L (2011) Correlation between rheological properties, in vitro release, and percutaneous permeation of tetrahydropalmatine. AAPS PharmSciTech 12:1002–1010
Li L, He M, Fang C, Zhang Y, Wang Y, Song X, Zou Y, Jia R, Liang X, Yin L (2022) Preparation, characterization, ex vivo transdermal properties and skin irritation evaluation of 1, 8-cineole nanoemulsion gel. Int J Pharm 624:121982
Mahuli M, Anandamoy R (2020) Development and formulation of aloe vera emulgel. GSC Biol Pharm Sci 12(2):161–166
Malan S, Chetty D, Du Plessis J (2002) Physicochemical properties of drugs and membrane permeability. S Afr J Sci 98(7):385–391
Matharoo NS, Garimella HT, German C, Przekwas AJ, Michniak-Kohn B (2023) A comparative evaluation of desoximetasone cream and ointment formulations using experiments and in silico modeling. Int J Mol Sci 24(20):15118
Mathur V, Satrawala Y, Rajput MS (2010) Physical and chemical penetration enhancers in transdermal drug delivery system. Asian J Pharm 4(3)
Modi D, Jonnalagadda S, Campbell GA, Dalwadi G (2023) Enhancing oil solubility of BCS class II drug phenytoin through hydrophobic ion pairing to enable high drug load in injectable nanoemulsion to prevent precipitation at physiological pH with a potential to prevent phlebitis. J Pharm Sci 112:2427–43
Moghimi HR, Shirazi FH, Ardestani MS, Oghabian MA, Saffari M, Sojoudi J (2016) In vitro and in vivo enhancement of antitumoral activity of liposomal antisense oligonucleotides by cineole as a chemical penetration enhancer. J Nanomater 16(1):345–345
Moser K, Kriwet K, Naik A, Kalia YN, Guy RH (2001) Passive skin penetration enhancement and its quantification in vitro. Eur J Pharm Biopharm 52(2):103–112
Nokhodchi A, Shokri J, Dashbolaghi A, Hassan-Zadeh D, Ghafourian T, Barzegar-Jalali M (2003) The enhancement effect of surfactants on the penetration of lorazepam through rat skin. Int J Pharm 250(2):359–369
Parhi R, Goutam SVS, Mondal S (2020) Formulation and evaluation of transdermal gel of ibuprofen: use of penetration enhancer and microneedle. Iran J Pharm Sci 16:11–32
Parikh KJ, Sawant KK (2019) Solubilization of vardenafil HCl in lipid-based formulations enhances its oral bioavailability in vivo: a comparative study using Tween-20 and Cremophor-EL. J Mol Liq 277:189–199
Patel MR, Patel MH, Patel RB (2016) Preparation and in vitro/ex vivo evaluation of nanoemulsion for transnasal delivery of paliperidone. Appl Nanosci 6(8):1095–1104
Patel H, Palekar S, Patel A, Patel K (2023) Ibrutinib amorphous solid dispersions with enhanced dissolution at colonic pH for the localized treatment of colorectal cancer. Int J Pharm 641:123056
Patel MR, Patel RB (2021) Nanoemulsion for topical therapy of acne: optimization and evaluation. Indian Drugs 58(2)
Puri V, Savla R, Chen K, Robinson K, Virani A, Michniak-Kohn B (2022) Antifungal nail lacquer for enhanced transungual delivery of econazole nitrate. Pharmaceutics 14(10):2204
Rathod VR, Shah DA, Dave RH (2020) Systematic implementation of quality-by-design (QbD) to develop NSAID-loaded nanostructured lipid carriers for ocular application: preformulation screening studies and statistical hybrid-design for optimization of variables. Drug Dev Ind Pharm 46(3):443–455
Romana-Souza B, Saguie BO, de Almeida Nogueira NP, Paes M, dos Santos Valença S, Atella GC, Monte-Alto-Costa A (2020) Oleic acid and hydroxytyrosol present in olive oil promote ROS and inflammatory response in normal cultures of murine dermal fibroblasts through the NF-κB and NRF2 pathways. Food Res Int 131:108984
Roy N, Agrawal M, Chaudhary S, Tirkey V, Dhwaj A, Mishra N (2017) Review article on permeation enhancers: a major breakthrough in drug delivery technology. Int J Pharm Sci Res 8(3):1001
Saini S, Baghel S, Chauhan S (2014) Recent development in penetration enhancers and techniques in transdermal drug delivery system. J Adv Pharm Educ Res 4(1):173–183
Saitoh H, Takami K, Ohnari H, Chiba Y, Ikeuchi-Takahashi Y, Obata Y (2023) Effects and mode of action of oleic acid and Tween 80 on skin permeation of disulfiram. Chem Pharm Bull 71(4):289–298
Salimi A, Emam M, Mohammad Soleymani S (2021) Increase adapalene delivery using chemical and herbal enhancers. J Cosmet Dermatol 20(9):3011–3017
Shah HG, Rathod V, Basim P, Gajera B, Dave RH (2022) Understanding the impact of multi-factorial composition on efficient loading of the stable ketoprofen nanoparticles on orodispersible films using Box-Behnken design. J Pharm Sci 111(5):1451–1462
Sharma N, Sharma V, Choukse R, Patel R (2020) Formulation and evaluation of ketoprofen gel. J Innov Invent Pharm Sci 1(1):22
Shi J, Cong W-J, Wang Y-M, Liu Q-F, Luo G-A (2013) Synergistic effect and mechanism of cineole and terpineol on in-vitro transdermal delivery of huperzine A from microemulsions. Iran J Pharm Res 12(2):271
Shokri N, Javar HA, Khalaj A (2014) Effect of hydrophilic solvents on enhancing transdermal delivery of albumin. Int J Pharm Sci Res 5(8):3175
Sliva J (2021) The importance of penetration enhancers choice in topical products. Klin Farmakol Farm 35(4):126–129
Supe S, Takudage P (2021) Methods for evaluating penetration of drug into the skin: a review. Skin Res Technol 27(3):299–308
Trommer H, Neubert R (2006) Overcoming the stratum corneum: the modulation of skin penetration: a review. Skin Pharmacol Physiol 19(2):106–121
Vardy D, Barenholz Y, Naftoliev N, Klaus S, Gilead L, Frankenburg S (2001) Efficacious topical treatment for human cutaneous leishmaniasis with ethanolic lipid amphotericin B. Trans R Soc Trop Med Hyg 95(2):184–186
Vig K, Chaudhari A, Tripathi S, Dixit S, Sahu R, Pillai S, Dennis VA, Singh SR (2017) Advances in skin regeneration using tissue engineering. Int J Mol Sci 18(4):789
Virani A, Dholaria N, Matharoo N, Michniak-Kohn B (2023a) A study of microemulsion systems for transdermal delivery of risperidone using penetration enhancers. J Pharm Sci 112:3109–3119
Virani A, Puri V, Mohd H, Michniak-Kohn B (2023b) Effect of penetration enhancers on transdermal delivery of oxcarbazepine, an antiepileptic drug using microemulsions. Pharmaceutics 15(1):183
Williams AC, Barry BW (2012) Penetration enhancers. Adv Drug Deliv Rev 64:128–137
Wortmann G, Zapor M, Ressner R, Fraser S, Hartzell J, Pierson J, Weintrob A, Magill A (2010) Lipsosomal amphotericin B for treatment of cutaneous leishmaniasis. Am J Trop Med Hyg 83(5):1028
Zhang A, Jung E-C, Zhu H, Zou Y, Hui X, Maibach H (2017) Vehicle effects on human stratum corneum absorption and skin penetration. Toxicol Ind Health 33(5):416–425
Author information
Authors and Affiliations
Contributions
Conceptualization, A.V.; methodology, A.V.; software, A.V.; validation, A.V. and B.M.-K.; formal analysis, A.V.; investigation, A.V.; resources, B.M.-K.; data curation, A.V.; writing—original draft preparation, A.V.; writing—review and editing, A.V., N.D., N.A., H.M. and B.M.-K.; cytotoxicity study, H.N.; visualization, A.V.; supervision, B.M.-K.; project administration, A.V. and B.M.-K.; funding acquisition, B.M.-K. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Availability of data and materials
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Funding
The support for this study was provided by the Center for Dermal Research CDR at the Rutgers, the State University of New Jersey, Piscataway, NJ, USA.
Acknowledgements
This study was funded by the Center for Dermal Research CDR, Rutgers-The State University of New Jersey, 145, Bevier Road, Piscataway, NJ 08854, USA.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Virani, A., Dholaria, N., Mohd, H. et al. Effect of chemical penetration enhancers on the transdermal delivery of olanzapine in human skin in vitro. AAPS Open 10, 4 (2024). https://doi.org/10.1186/s41120-024-00092-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41120-024-00092-1