Abstract
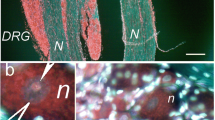
Immunohistochemical studies were performed to address the expression of the high-molecular-weight component of the neurofilament triplet NF200 (a marker of neurons forming A fibers) and the binding of isolectin B4 (IB4) by neurons of the L4–5 spinal ganglia after ligation or section of the sciatic nerve in rats. A total of 15% of neurons in the ganglia of intact rats expressed NF200. By 90 days after nerve ligation, the proportion of NF200+ neurons decreased two-fold; administration to these rats of the nerve regeneration stimulator xymedone increased the number of NF200+ neurons by 50.7% compared with controls (ligation, no treatment). In intact rats, 23.6% of neurons bound IB4. The proportion decreased by 2.6% 30 days after nerve ligation and to undetectable levels by 90 days; xymedone increased the proportion of surviving IB4 + neurons more than eight-fold. IB4 + neurons were more likely to enter post-traumatic apoptosis. Ligation of the nerve was followed by survival of fewer NF200+ and IB4 + neurons than section of the nerve, which suggests that axon lengthening is a factor maintaining neuron survival. The pyrimidine derivative xymedone increased the survival of neurons of both subpopulations, especially IB4 + neurons.
Similar content being viewed by others
REFERENCES
I. S. Raginov, R. Kh. Khafiz'yanova, A. Yu. Vafin, and Yu. A. Che-lyshev, “The effects of the therapeutic agents xymedone and nootropil on peripheral nerve regeneration,” Ross. Morfol. Vedo-mosti, 1, No. 6, 120–126 (1997).
I. S. Raginov and Yu. A. Chelyshev, “Sensitive neurons and Schwann cells in pharmacological stimulation of nerve regeneration,” Morfologiya, 118, No. 6, 36–40 (2000).
M. Brown, E. Lunn, and V. Perry, “Consequences of slow Wallerian degeneration for regenerating motor and sensory axons,” J. Neurobiol., 23, No. 5, 521–536 (1992).
H. Hammarberg, F. Piehl, S. Cullheim, et al., “GDNF mRNA in Schwann cells and DRG satellite cells after chronic sciatic nerve injury,” Neuroreport, 22, 857–860 (1996).
D. Henken, W. Battisti, and M. Chesselet, “Expression and beta-pre-protachykinin mRNA and tachykinins in rat dorsal root ganglion cells following peripheral or central axotomy,” Neurosci., 39, No. 3, 733–742 (1990).
H. Kashiba, Y. Uchida, and E. Senba, “Difference in binding by isolectin B4 to trkA and c-ret mRNA-expressing neurons in rat sen-sory ganglia,” Mot. Brain Res., 95, No. 1–2, 18–26 (2001).
E. Kinnman, H. Aldskogius, O. Johansson, and Z. Wiesenfeld-Hallin, “Collateral reinnervation and expansive regenerative reinnervation by sensory axons into 'foreign' denervated skin: An immunohistochemical study in the rat,” Exptl. Brain Res., 91, No. 1, 61–72 (1992).
P. Leclerc, P. Ekstrom, A. Edstrom, et al., “Effects of glial cell line-derived neurotropic factor on axonal growth and apoptosis in adult mammalian sensory neurons in vitro,” Neurosci., 82, 545–558 (1997).
Q. Ma, “Vanilloid receptor homologue, VRL1, is expressed by both A-and C-fiber sensory neurons,” Neuroreport, 12, No. 17, 3693–3695 (2001).
D. Molliver, D. Wright, M. Leitner, et al., “IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life,” Neuron, 19, 4849–4861 (1997).
J. Nagy and S. Hunt, “Fluoride-resistance acid phosphatase-containing neurones in dorsal root ganglia are separate from those containing substance P or somatostatin,” Neurosci., 7, 89–97 (1982).
J. Petruska, J. Japaporn, R. Johnson, et al., “Subclassified acutely dissociated cells of rat DRG: histochemistry and patterns of cap-saicin-, proton-, and ATP-activated currents,” J. Neurophysiol., 84, No. 5, 2365–2379 (2000).
M. Ramer, J. Priestley, and S. McMahon, “Functional regeneration of sensory axons into adult spinal cord,” Nature, 403, 312–316 (2000).
J. Schionning, J. Larsen, T. Tandrup, and H. Braendgaard, “Selective degeneration of dorsal root ganglia and dorsal nerve roots in methyl mercury-intoxicated rats: a stereological study,” Acta Neuropathol., 961, 191–201 (1998).
J. Silverman and L. Kruger, “Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reac-tivity to peptide and enzyme markers,” J. Neurocytol., 19, 789–801 (1990).
T. Tandrup, C. Woolf, and R. Coggleshall, “Delayed loss of small dorsal root ganglion cells after transection of the rat sciatic nerve,” J. Comp. Neurol., 422, No. 2, 172–180 (2000).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Raginov, I.S., Chelyshev, Y.A. Post-Traumatic Survival of Sensory Neurons of Different Subpopulations. Neurosci Behav Physiol 35, 17–20 (2005). https://doi.org/10.1023/B:NEAB.0000049647.19397.30
Issue Date:
DOI: https://doi.org/10.1023/B:NEAB.0000049647.19397.30