Abstract
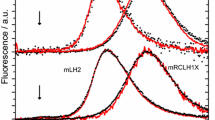
Low-temperature spectrophotometry was used to study the primary stages of rhodopsin photolysis. A digitonin extract of rhodopsin was irradiated at –155°C with blue light of wavelength 436 nm. The stage of the bathorhodopsin → lumirhodopsin conversion was accompanied by the simultaneous formation of several products. Formation of an intermediate product spectrally similar to the known “blue-shifted intermediate” (BSI) was demonstrated. It is suggested that the appearance of more than one intermediate product at each stage of photolysis reflects the existence of several conformational states of the rhodopsin molecule during its photoconversion.
Similar content being viewed by others
REFERENCES
Kh. Kandori, I. Shichida, and T. Ioshizava, “Photoisomerization of rhodopsin. A Review,” Biokhimiya, 66, No. 11, 1484–1498 (2001).
V. A. Krongauz, R. R. Shifrina, I. B. Fedorovich, and M. A. Ostrovksii, “Photochromia of visual pigments. 1. Formation of isochromic products by reversible conversions of frog rhodopsin,” Biofizika, 20, No. 2, 219–224 (1975).
T. B. Protasova, V. F. Tarasov, and I. B. Fedorovich, “The isomer composition of retinal in frog rhodopsin irradiated at 77 K," Sensor. Sistemy, 3, No. 1, 19–24 (1989).
C. M. Einterz, S. J. Hug, J. W. Lewis, and D. S. Kliger, “Early photolysis intermediates of the artificial visual pigment 13-demethylrhodopsin,” Biochemistry, 29, No. 6, 1485–1491 (1990).
C. M. Einterz, J. W. Lewis, and D. S. Kliger, “Spectral and kinetic evidence for the existence of two forms of bathorhodopsin,” Proc. Natl. Acad. Sci. USA, 84, 3699–3703 (1987).
K.-H. Grellmann, R. Livingston, and D. Pratt, “A flash-photolytic investigation of rhodopsin at low temperatures,” Nature, 193, 1258–1260 (1962).
S. J. Hug, J. W. Lewis, C. M. Einterz, T. E. Thorgeirsson, and D. S. Kliger, “Nanosecond photolysis of rhodopsin: evidence for a new, blue-shifted intermediate,” Biochemistry, 29, No. 5, 1485–1486 (1990).
S. J. Hug, J. W. Lewis, and D. S. Kliger, “Evidence for common batho intermediate of rhodopsin and isorhodopsin,” J. Amer. Chem. Soc., 110, 1998–1999 (1988).
S. Jager, J. W. Lewis, T. A. Zvyaga, I. Szundi, T. P. Sakmar, and D. S. Kliger, “Chromophore structural changes in rhodopsin from nanoseconds to microseconds following pigment photolysis,” Proc. Natl. Acad. Sci. USA, 94, 8557–8562 (1997).
J. W. Lewis and D. S. Kliger, “Absorption spectroscopy in studies of visual pigments: spectral and kinetic characterization of intermediates,” Meth. Enzymol., 315, 164–178 (2000).
L. A. Peteanu, R. W. Schoenlein, Q. Wang, R. A. Mathis, and C. V. Shank, “The first step in vision occurs in femtoseconds: complete blue and red spectral studies,” Proc. Natl. Acad. Sci. USA, 90, 11762011766 (1993).
T. B. Protasova, I. V. Fedorovich, and M. A. Ostrovsky, “Retinals isomers in photo-and electro-induced rhodopsin intermediates,” Light in Biology and Medicine, 2, 545–555 (1992).
N. Sasaki, F. Tokunaga, and T. Yoshizawa, “The formation of two forms of bathorhodopsin and their optical properties,” Photochem. Photobiol., 32, 433–441 (1980).
H. G. Smith, G. W. Stubb, and B. J. Litman, “The isolation and purification of osmotically intact discs from retinal rod outer segments,” Exptl. Eye Res., 20, 211–217 (1975).
J. G. Stewart, B. N. Baker, and T. P. Williams, “Kinetic evidence for a conformational transition in rhodopsin,” Nature, 258, 89–90 (1975).
L. Ujj, F. Jager, and G. H. Atkinson, “Vibrational spectrum of the lumi intermediate in the room temperature rhodopsin photo-reaction,” Biophys. J., 74, No. 3, 1492–1501 (1998).
Q. Wang, R. W. Schoelein, L. A. Peteanu, R. A. Mathis, and C. V. Shank, “Femtosecond dynamics of the cis-trans isomerization in rhodopsin: the first step in vision,” in: Thirty-Seventh Annual Meeting of the Biophysical Society, Washington DC (1993), p. A127.
T. Yoshizawa, Y. Shichida, and S. Matuoka, “Primary intermediates of rhodopsin studied by low temperature spectrophotometry and laser photolysis,” Vision. Res., 24, No. 11, 1455–1463 (1984).
T. Yoshizawa and G. Wald, “Prelumirhodopsin and the bleaching of visual pigments,” Nature, 197, 1279–1285 (1963).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fel'dman, T.B., Fedorovich, I.B. & Ostrovskii, M.A. Characteristics of the Photoconversion of Rhodopsin in the Early Stages of Photolysis. Neurosci Behav Physiol 34, 735–742 (2004). https://doi.org/10.1023/B:NEAB.0000036015.85880.9b
Issue Date:
DOI: https://doi.org/10.1023/B:NEAB.0000036015.85880.9b