Abstract
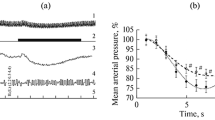
Chronic experiments were performed on cats to study the effects of electrical stimulation of the medial preoptic area of the hypothalamus on the latent period, duration, and structure of paradoxical sleep, as well as the dynamics of neuron activity in this structure during the sleep-waking cycle. These investigations showed that low-frequency stimulation of the medial preoptic area during slow-wave sleep led to short-latency development of desynchronization of bioelectrical activity in the neocortex and initiated the development of paradoxical sleep or a similar state. Stimulation of this structure during paradoxical sleep led to a decrease in its duration, to the virtually complete disappearance of the tonic stage of paradoxical sleep, and to an increase in the frequency of rapid eye movements in the phasic stage. Rearrangement of neuron activity in the medial preoptic area during the sleep-waking cycle was similar to that seen in cells of the lower brainstem “executive” centers of paradoxical sleep. It is suggested that neurons in the medial preoptic area of the hypothalamus are actively involved in the mechanisms of paradoxical sleep and, in particular, in the desynchronization of neocortical bioelectrical activity which develops during this stage.
Similar content being viewed by others
REFERENCES
L. P. Latash, “The neurophysiology of sleep and dreams,” in: Clinical Neurophysiology [in Russian], Nauka, Leningrad (1972), p. 215.
T. N. Oniani, M. G. Koridze, M. G. Kavkasidze, and L. B. Gvetadze, “The effects of electrical stimulation of the mid-and intermediate brain of the dynamics of different phases of sleep and their relationships,” in: The Neurophysiology of Emotions and the Sleep-Waking Cycle [in Russian], Metsniereba, Tbilisi (1976), p. 28.
L. A. Popova, Mechanisms of the Daily Periodicity of Waking and Sleep [in Russian], Author's abstract of thesis for doctorate in biological sciences, Leningrad State University, Leningrad (1966).
N. V. Suntsova and O. Yu. Dergacheva, “Forebrain mechanisms for the regulation of paradoxical sleep,” in: Progress in Neurocybernetics. Proceedings of the XII International Conference, Rostov on Don (1999), p. 52.
M. Alam and B. Mallick, “Differential acute influence of medial and lateral preoptic areas on sleep-wakefulness in freely moving rats,” Brain Res., 525, 242 (1990).
S. Asala, Y. Okano, K. Honda, et al., “Effects of medial preoptic area lesions on sleep and wakefulness in unrestrained rats,” Neurosci. Lett., 114, 300 (1990).
G. Benedek, F. Obal, L. Szekeres, et al., “Two separate synchronizing mechanisms in the basal forebrain: study of the synchronizing effects of the rostral hypothalamus, preoptic region and olfactory tubercle,” Arch. Ital. Biol., 117,No. 2, 164 (1979).
O. Candia, E. Favale, A. Giussani, et al., “Blood pressure during natural sleep and during sleep induced by electrical stimulation of the brain stem reticular formation,” Arch. Ital. Biol., 100, 216 (1962).
J. Faure, C. Bensch, and D. Vincent, “Role d'un systeme mesencephalo-limbique dans la ‘phase paradoxale’ du sommeil chez le lapin,” Compt. Rend. Soc. Biol., 156, 70 (1962).
H. Jasper and C. Ajmone-Marsan, A Stereotaxic Atlas of the Diencephalon of the Cat, National Research Council of Canada, Ottawa (1954).
J. John and V. Kumar, “Effect of NMDA lesion of the medial preoptic neurons on sleep and other functions,” Sleep, 21, 587 (1998).
M. Jouvet, “Recherches sur les structures nerveuses et les mecanismes responsables des differentes phases du sommeil physiologique,” Arch. Ital. Biol., 100, 125 (1962).
M. Jouvet, “Etude de la dualite des etats de sommeil et des mecanismes de la phase paradoxale,” in: Aspects Anatomo-functionels de la Physiologie de Sommeil, CNRS, Paris (1965), p. 393.
M. Jouvet, “The regulation of paradoxical sleep by the hypothalamo-hypophysis,” Arch. Ital. Biol., 126,No. 4, 259 (1988).
K. Kaitin, “Preoptic area unit activity during sleep and wakefulness in the cat,” Exptl. Neurol., 83,No. 2, 347 (1984).
J. Koyama and O. Hahaishi, “Firing of neurons in the preoptic/anterior hypothalamic areas in rat: its possible involvement in slow wave sleep and paradoxical sleep,” Neurosci. Res., 19, 31 (1994).
M. Mansari, K. Sakai, and M. Jouvet, “Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats,” Exptl. Brain Res., 76, 519 (1989).
D. McGinty and M. Sterman, “Sleep suppression after basal forebrain lesions in the cat,” Science, 160,No. 3833, 1253 (1968).
H. Onoe and K. Sakai, “Kainate receptors: a novel mechanism in paradoxical (REM) sleep generation,” Neuroreport, 6, 353 (1995).
P. Passouant and J. Cadilhac, “Les rhythmes theta hippocampiques au cours de sommeil,” in: Physiologie de L'Hippocampe, CNRS, Paris (1962), p. 331.
Z. Rao, M. Yamano, and A. Wanaka, “Distribution of cholinergic neurons and fibers in the hypothalamus of the rat using choline acetyltransferase as a marker,” Neurosci., 20, 923 (1987).
E. Roldan, T. Weiss, and E. Fifkova, “Excitability changes during the sleep cycle of the rat,” EEG Clin. Neurophysiol., 15, 775 (1963).
K. Sakai, “Executive mechanisms of paradoxical sleep,” Arch. Ital. Biol., 126,No. 4, 239 (1988).
K. Sakai and D. Salvet, “Cholinergic and monoaminergic afferents to pontine paradoxical sleep executive region in the cat,” J. Sleep Res., 5, 201 (1996).
M. Sallanon, M. Denoyer, K. Kitahama, et al., “Long-lasting insomnia induced by preoptic neuron lesions and its transient reversal by muscimol injection into the posterior hypothalamus in the cat,” Neurosci., 32,No. 3, 669 (1989).
P. Saxena, K. Tangri, and K. Bhargava, “An experimental study of the synchronizing system in the brain,” EEG Clin. Neurophysiol., 17, 506 (1964).
S. Torii, “Two types of pattern of hippocampal electrical activity induced by stimulation of hypothalamus and surrounding parts of rabbit's brain,” Jap. J. Physiol., 11, 147 (1961).
R. Ursin and M. Sterman, A Manual for Recording and Scoring of Sleep Stages in the Cat, Brain Information Service, Brain Research Institute, Los Angeles (1981).
T. Yokota and B. Fujimori, “Effects of brain-stem stimulation upon hippocampal electrical activity, somatosensory reflexes, and autonomic functions,” EEG Clin. Neurophysiol., 16, 375 (1964).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Suntsova, N.V., Dergacheva, O.Y. The Role of the Medial Preoptic Area of the Hypothalamus in Organizing the Paradoxical Phase of Sleep. Neurosci Behav Physiol 34, 29–35 (2004). https://doi.org/10.1023/B:NEAB.0000003243.95706.de
Issue Date:
DOI: https://doi.org/10.1023/B:NEAB.0000003243.95706.de