Abstract
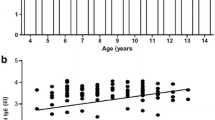
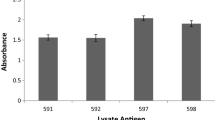
The only report hitherto, from India in 1982, on anti-rhinosporidial antibody levels in patients with rhinosporidiosis recorded that antibody was not detected in Indian patients. The present report describes the use of the dot-ELISA assay of serum anti-rhinosporidial IgG, IgM and IgA and salivary sIgA in patients with diverse clinical presentations, in rural asymptomatic persons who had bathed in ground waters that probably harboured the causative pathogen, Rhinosporidium seeberi, and in laboratory persons who were exposed to R. seeberi. Ultrasonic extracts of purified endospores and sporangia of R. seeberi were used as antigen. The geometric mean (reciprocal) titres of serum antibody detected in patients were IgM 142.1, IgG 178.5, IgA 84.6, with ranges of 0–640, 30–960 and 0–160 respectively, salivary sIgA titres ranged from 0 to 18 with a mean of 4.6. The levels of antibody had no correlation with the site, the number of sporangia, duration and recurrence of the disease. Asymptomatic persons from the same endemic area as patients showed mean titres of IgM 89.6, IgG 69.1, 1gA 95.5, with salivary sIgA titres of 3.1. Asymptomatic personnel who had been working in a laboratory where rhiniosporidial work was being done, showed mean titres of 169.6 IgM, 62.8 IgG, and 6.5 salivary sIgA. These results indicate that an anti-rhinosporidial antibody response occurs in rhinosporidial patients, as well as in asymptomatic persons who were exposed to R. seeberi in the environment. Anti-R. seeberi antibody does not appear to be protective in rhinosporidiosis since appreciable titres were present in patients with recurrent, single, multiple or disseminated lesions of long duration.
Similar content being viewed by others
References
Chitravel V, Sundararaj T. Detection of circulating antigen in patients with rhinosporidiosis. Sabouraudia 1982;20:185–191.
Atapattu DN, Arseculeratne SN, Rajapakse RPVJ, Perera NAND, Eriyagama NB Purification of the endospores and sporangia of Rhinospondium seeberi on Percoll columns. Mycopathologia 1999;145:113–119.
Atapattu DN, Arseculeratne SN, Rajapakse RPVJ, Perera NAND, Razak MAA Is human IgG bound by endospores and sporangia of Rhinosporidium seeberi ?–a possible mechanism of immune evasion. Proc Kandy Soc Med,2000;22:34.
Ledue TB, Garfin DE. Immunoxation and immunoblotting. In Rose NR, De Macario EC, Fold JD, Lane HC, Nakamura RM,eds. Manual of clinical immunology. Washington DC: ASM Press,1997;21:58–64.
Lu Huaguang. A longitudinal study of a novel dot-enzyme-linked immunosorbent assay for detection of Avian lnfluenza. Virus 2002;47(2):361–369.
Sasty KSR, Tuteja U, Santhosh PK, Lalitha MK, Batra HV. Identification of Bacillus anthracis by a simple protective antigen-speci c mAb dot-ELISA. J Med Microbiol 2003;52:47–49.
Branch SL, Levett PN. Evalution of four methods for detection of immunoglobulin M antibodies to dengue virus. Clin Diag Lab Immunol 1999;6(4):555–557.
Londner MV, Revel A, Rosen G, Shehata MG, Kemawi MA, el-Said S, Spira T. Dot-ELISA, a potential immunoassay for the detection of Plasmodium falciparum antibodies.Trans Roy Soc Trop Med Hyg 1988;82(5):686–688.
Gürtürk K. Ekin IH, Aksakal A, Solmaz H. Detection of Campylobacter antibodies in sheep sera by a Dot-ELISA using acid extracts from C.fetus ssp.and C.Jejuni strains and comparison with a complement xation test. J Vet Med B 2002;49:146–151.
Jayasekera S, Arseculeratne SN. Atapattu DN, Kumarasiri R. Tilakaratna WM. Cell-mediated immune responses (CMIR)to Rhinosporidium seebari in mice. Mycopathologia 2001;152:69–79.
De Silva NR, Huegel H, Atapattu DN, Arseculeratne SN, Kumarasiri R, Gunawardena S, Balasooriya P, Fernando R. Cell-mediated immune responses in human rhinosporidiosis. Mycopathologia 2001;152:59–68.
Arseculeratne SN. Recent advances in rhinosporidiosis and Rhinosporidium seeberi. Indian J Med Microbiol 2002;20(3):119–131.
Appuhamy S, Atapattu DN, Arseculeratne SN, Eriyagama NB. Strain variation in Rhinosporidium Seeberi, Proceedings of the Sri Lanka College of Microbiologists 2002;OP7.
Arseculeratne SN, Ajello L. Rhinosporidium seeberi.ln: Ajello L, Hay RJ,eds.Topley & Wilson 's microbiology</del>and microbial infections, Medical Mycology, Vol.4, 9th edn, London: Arnold,1998.
Arseculeratne SN. The effect of biocides (antiseptics and disinfectants)on the endospores of Rhinosporidium seeberi. Proceedings of the Sri Lanka College of Microbiologists2002;OP8.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arseculeratne, S., Kumarasiri, P., Rajapakse, R. et al. Anti-Rhinosporidial Antibody Levels in Patients with Rhinosporidiosis and in Asymptomatic Persons, in Sri Lanka. Mycopathologia 158, 157–164 (2004). https://doi.org/10.1023/B:MYCO.0000041903.48717.79
Issue Date:
DOI: https://doi.org/10.1023/B:MYCO.0000041903.48717.79