Abstract
Background
To evaluate total and specific E immunoglobulin (IgE) antibody concentrations in underage subjects with respiratory allergic diseases.
Methods
This study was a transversal-type study in 100 underage subjects between 4 and 14 years old, with asthma and/or allergic rhinitis. Total and specific IgE were quantified for airborne fungi in the city of São Luís, Maranhão, Brazil. Five distinct regions—North, South, Center, East and West—were selected so fungi could be collected monthly for 1 year. Twenty genera were identified. Aspergillus, Penicillium, Fusarium and Neurospora were selected for the preparation of sensitizing antigens from ELISA dishes. IgE total concentrations were estimated using the same method.
Results
IgE total serum concentration was increased in 97 % of the atopic subjects: 75 % of the subjects presented increased IgE anti-Aspergillus concentrations, 87 % presented IgE anti-Penicillium, 45 % presented IgE anti-Fusarium, and 46 % presented IgE anti-Neurospora.
Conclusions
Atopic subjects presented simultaneous IgE total and specific elevations for the tested fungi, possibly due to polysensitization caused by the presence of fungi in all of the areas all year. However, determining the clinical significance of the results was not yet possible because most of the data were isolated variables.
Similar content being viewed by others
Background
Respiratory disorders are higher prevalent in children. The different manifestations of allergic diseases involve the upper and lower respiratory airways.
Fungi are important pathogens associated with airway diseases. They interact with epithelial cells, induce increases in inflammatory cytokines, which enhance IgE production, and increase toll-like receptor (TLR) expression [1, 2]. Exposure to fungi can have adverse effects on human health through serious immune responses, infections of several organs and the irritating toxic effects of their subproducts [3].
Fungi and fungal-like organisms are the cause of a lot of diseases in humans and animals, but are also economically important [4]. Alternaria, Aspergillus, Cladosporium, Fusarium, Penicillium, Stachybotrys are producers of mycotoxins and allergens that cause respiratory diseases [5].
Allergic rhinitis and allergic asthma are characterized as type 1 allergy. Type 1 allergy is caused by a large number of fungi [6]. Allergic rhinitis is characterized by frequent episodes of sneezing, rhinorrhea, pruritus and nasal obstructions. It is induced by a large number of fungal species, with Alternaria, Aspergillus, Bipolaris, Cladosporium, Curvularia and Penicillium being the most prominent [6].
Allergic asthma is a respiratory disease characterized by exposure to environmental agents, which provoke allergic inflammation and transitory bronchiolar obstruction, resulting in typical symptoms of coughing and dyspnea [7]. In children, studies conducted by Halonen et al. [8], Nelson et al. [9] and Peat et al. [10] demonstrated that fungal allergy was shown to be associated with increased bronchial reactivity. The genera Alternaria, Aspergillus, Cladosporium, Helminthosporium, Epicoccum, Aureobasidium and Penicillium have frequently been implicated in allergic asthma [11–13].
Before the outcomes of experimental models, asthma was considered to be a disease caused by IgE because it only occurred due to antigen–antibody complexes formed in mastocyte membranes [12]. Most recent studies have shown that T cells actively participate in asthma manifestation and can cause airway obstruction and allergic inflammation due to the release of cytokines, such as IL-4 and IL-13, which are associated with mastocyte and eosinophil activation [13, 14]. Elevated IgE is commonly found not only in allergic manifestations but also in intestinal and cutaneous parasitosis, acquired or congenital immunodeficiency, viral infections and neoplasias [15, 16]. Also, asthma risk is influenced by genetics. Having a parent with asthma doubles a child’s risk of asthma, and having 2 affected parents increases the risk fourfold. [17].
Indoor dampness increases the risk of indoor fungal growth. The indoor fungal concentrations and species diversity could improve the risk of having asthma, exacerbation of asthma symptoms, or both. Penicillium and Aspergillus species propagules have been shown to increase the risk of asthma development in children. [18, 19].
In general, outdoor fungi are generally more abundant than indoor fungi, but the association between outdoor fungi and child asthma exacerbations has not been clearly established [20]. The fungal species Alternaria, Aspergillus, Aureobasidium, Cladosporium, Epicoccum, Helminthosporium and Penicillium, are most frequently implicated in fungi-related allergic asthma exacerbations amongst adults in indoor and outdoor settings [6].
In Brazil, Mezarri et al. [21] found a higher prevalence of the fungi Cladosporium (17.86 %) and Aspergillus/Penicillium (15.03 %) in the atmospheric air of Porto Alegre, similar to other countries, such as France, Chile, USA and Cuba. Mendes et al. [22] mainly identified Aspergillus, Botryodiplodia and Curvularia in Aracaju.
The current research aimed to isolate and extract the most common airborne antigens to detect specific IgE concentrations in the serum of atopic subjects, and the expectation was that these concentrations would be correlated according to the patients’ sex, age and place of residence. By doing so, the findings could actually contribute to better control of the disease.
Methods
Area of research
The island of São Luís, which is the capital of Maranhão State, Brazil, is in the center of the Maranhão seashore, at 2°20′00′′S to 2°45′00′′S and longitude 44°01′21′′W to 44°24′54′′W. It is located in the north of the state, bordering the Atlantic Ocean; it stretches south to Estreito dos Mosquitos, which separates it from the continent, east to São José Bay and west to São Marcos Bay. It features hot and humid weather and a steady temperature around 27–33 °C [23].
Sample calculation
To calculate the sample size, Epi Info 3.4.2 (2007, Atlanta, GA) statistical software was used. The calculation was based on the prevalence of asthma in the reference service at 5 % significance.
Patients
The study sample consisted of 100 patients (64 % boys and 36 % girls), between 4 and 14 years old enrolled in the Pediatric Pneumology Program, University Hospital, Federal University of Maranhão (HUUFMA). Through-chip protocol, clinical data were obtained from patients in the period from 2007 to 2008.
From these subjects, 10 ml of total blood was collected by intravenous puncture after the parent’s/tutors signed the instrument of free consent. The serum was separated and aliquoted for storage in a freezer at 20 °C until the moment of performing the techniques.
Isolation and airborne fungi identification
Airborne fungi were monthly collected by Bezerra [24], in five areas (North, South, Center, East and West) in the city of São Luís, Maranhão, Brazil, from January to December 2007.
Fungal spores were collected by passive gravitational settling on Petri dishes containing Sabouraud agar, 1.5 m height, for 15 min [21, 25]. A total of 1510 colony forming units (CFUs) were recovered, corresponding to 20 fungi genera. The species will be identified later by microculture technique and electronic microscopy.
Only the genera Aspergillus, Penicillium, Fusarium and Neurospora showed detectable amounts of protein and were used as antigens to sensitize ELISA plates to investigate the concentration of specific IgE by ELISA index (EI) [26].
Antigenic extraction
The protocol for the extraction of antigens from filamentous fungi was performed as follows, based on the literature [27, 28] with certain modifications: the fungi were cultivated in an appropriate medium (Sabouraud ágar) for 5 days of culture before washing five times with phosphate-buffered saline (PBS) and transferring the culture to screw-cap tubes containing previously sterilized glass beads and PBS and fungus at a ratio of 3:1. The tubes were vortexed for 15 min and centrifuged for 1 h at 14,500 rpm in a refrigerated centrifuge The supernatant was collected, and the protein was quantitated. The stock of crude extract was aliquoted and stored at 20 °C until use. Stocks were diluted to 50 µl/ml in PBS before use.
The sera from 100 patients with clinically characterized respiratory diseases were subjected to ELISA to detect the concentrations of total and specific IgE against fungi.
Polystyrene plates with 96 wells containing immobilized monoclonal antibodies of the anti-IgE type formed specific complexes with serum IgE through high-affinity bonds. The plates were then washed to remove proteins that had not interacted. A secondary antibody conjugated to peroxidase was added to form a ternary complex with the immobilized.
IgE, followed by washing. Next, 3-30,5-50-tetramethylbenzidine and hydrogen peroxide were added to the solution, which under the action of the immobilized peroxidase, produced a blue color. A strong acid was added to the mixture to halt the reaction, producing a yellow color. A reading was performed at a wavelength of 450 nm. The absorbed intensity was proportional to the concentration of IgE in the sample.
Optical densities were determined spectrophotometrically at 450 nm. The rates of antibodies were expressed as the EI, according to Alves et al. [29]. The EI is calculated by the ratio between the mean optical density of the study group and the optical density of three samples of sera from subjects with no specific IgE (multiplied by 3d, where d is the average standard deviation of optical densities of sera from the group of non-allergic individuals).
EI values equal to or higher than 0.102 (EI ≥ 0.102) were considered to be positive for total IgE; 0.603 (EI ≥ 0.603) for IgE anti-Aspergillus spp.; 0.466 (EI ≥ 0.466) for IgE anti-Penicillium spp.; 0.298 (EI ≥ 0.298) for IgE anti-Neurospora spp.; and 0.565 (EI ≥ 0.565) for IgE anti-Fusarium spp.
The cut-off was calculated by averaging the increased negative samples three times to the standard deviation of these samples, where the number of standard deviations used in the formula ensures the confidence level of the result. Cut-off = μ DO + 3 × s [30].
Ethical aspects
This research was approved by the ethics committee of University Hospital under number 406/06. The Informed Consent Term (ICF) was signed by the parents or guardians of minors who participated in this research.
Statistical analysis
Statistical analysis was performed using the software Stata/SE 9.0 for Windows (Stata Corporation, College Station, TX). The study was cross-sectional and observational, and descriptive statistical techniques were used to assess all of the variables, with the aid of graphs and tables of frequencies. The Pearson correlation was used to study the relationship between total IgE levels and age (years). The correlation coefficient Phi was used to verify the correlation between positivity for two fungi concomitantly. We considered a p value <0.05 as the significance criterion.
Results
We studied 100 patients (67 % boys and 33 % girls), between 4 and 14 years old enrolled in the Pediatric Pneumology Program, University Hospital, Federal University of Maranhão (HUUFMA).
A total of 100 patients with a diagnosis of asthma were studied. In total, 45 (45.0 %) of these patients had rhinitis, and 35 (35.0 %) had sinusitis.
Table 1 shows the demographic data. Of the 100 patients studied, 67 (67.0 %) were male and 33 (33.0 %) were female. The mean age was 8.6 ± 2.6 years. In total, 100 % were from an urban area. In all, 19 patients (19.0 %) lived in the North Zone of São Luis; 6 (6.0 %) lived in the South Zone; 35 (35.0 %) lived in the East Zone; 17 (17.0 %) lived in the West Zone and 23 (23.0 %) lived in downtown area. Of the 59 children aged 4–9 years, 20 are female and 39 male, and of the 41 children in the age group 10–14 years, 13 are female and 28 male.
Observing Table 2, it is possible to observe that isolated fungus occurrences varied slightly over the year, considering the great rain variation between the rainy season (January–June) of 1702.25 mm3 and the dry season (July–December) of 35.50 mm3. The median number of CFUs/dish in the rainy season (January–June) was 20 (maximum = 279 and minimum = 0), and the corresponding figure for the dry season was 14 (maximum = 227 and minimum = 0) using the Mann–Whitney test p = 0.96.
Twenty genera of fungi were isolated in this study. The main genera found in all of the regions were Aspergillus (33.5 %), Penicillium (18.8 %), Cladosporium (14.2 %), Curvularia (10.6 %) and Fusarium (7.6 %). A comparison of the average number of CFUs for the five most frequent fungi using Tukey’s test, showed that the number for Aspergillus differed significantly (p < 0.05) from the numbers for the genera Cladosporium, Curvularia and Fusarium.
Aspergillus and Penicillium were frequently isolated in all of the areas studied, regardless of the month of the year. It was possible to see that, in the Central area, the lowest CFU number (115) was noted; in contrast, in the East area, the highest figures were measured (228) (Table 3).
In this study, we used the genders Aspergillus, Penicillium, Fusarium and Neurospora as antigens.
Detection of total and specific IgE for fungi antigens
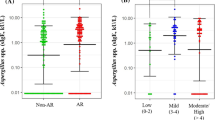
Specific IgE antibodies against fungi (anti-Neurospora, anti-Fusarium, anti-Aspergillus and anti-Penicillium IgE) were screened in the afore mentioned 100 blood samples. Among the 100 patients enrolled in the Pediatric Pneumology Program, 97 (97 %) demonstrated that IgE total concentrations were detected (Fig. 1a): 64 % boys and 33 % girls. There was a statistically significant difference in IgE total concentration and age (p < 0.05) (Fig. 1b), but not between IgE total concentration and area of residence (North, South, East, West and Center) (p = 0.88).
Among the 100 patients enrolled in the Pediatric Pneumology Program, 75 % were sensitized to Aspergillus, 87 % were sensitized to Penicillium; 46 % were sensitized to Neurospora and 45 % were sensitized to Fusarium.
Of the 59 children aged 4–9 years, 93.2 % tested positive for total IgE, 78 % tested positive for IgE anti-Neurospora, 76.2 % tested positive for IgE anti-Fusarium, 76.2 % tested positive for IgE anti-Aspergillus and 84.7 % tested positive for IgE anti-Penicillium. Of the 41 children in the age group 10–14 years, 100 % were positive for total IgE, 68.3 % tested positive for IgE anti-Neurospora, 68.3 % tested positive for IgE anti-Fusarium, 68.3 % tested positive for IgE anti-Aspergillus and 87.8 % tested positive for IgE anti-Penicillium.
Sex (p = 0.86), local of residence (p = 0.90) and age (p = 0.19) were not statistically significant with anti-Aspergillus IgE antibodies. There wasn’t a statistically significant difference in anti-Penicillium IgE antibodies and sex (p = 0.39), age (p = 0.47) and local of residence (p = 0.13).
Figure 2 shows the seropositivity to fungi of Total IgE, anti-Neurospora; anti-Fusarium; anti-Aspergillus and anti-Penicillium concentration IgE, in children´s serum. A prevalence of seropositivity to fungi was noted, particularly for the genera Aspergillus (Fig. 2).
Discussion
Fungi are important airborne allergens. Aspergillus spp. was the most commonly isolated genus in the current study, and Penicillium spp. was the second most commonly isolated genus. Aspergillus, Penicillium, Cladosporium, Curvularia and Fusarium are the most frequent outdoor species, according to previous researches [31, 32]. Research conducted in Mexico city showed that Penicillium spp. is also the second most common genus [33]. In Brazil, nostheast cities, like Fortaleza, Natal and Recife are climatically similar with São Luís. In Recife and Natal, Aspergillus and Penicillium were more frequente genera [34, 35]. In Fortaleza, Curvularia spp. was the most isolated fungi [25].
IgE antibody concentrations in serum vary according to age and as a consequence of contact with antigens, which is also common among the other immunoglobulin classes. In this research, these concentrations were higher in boys regardless of age. Spalding et al. [36] demonstrate that the concentration of IgE is higher for males in all age groups. Our results corroborate these authors, since the increase of IgE occurred in 64 % of males and 33 % females.
Specific IgE dosing is one of the most important in vitro methods for the diagnosis of immediate hypersensitivity [37]. In the current research, increased IgE concentrations were observed in 96.9 % of the patients. These figures were higher than those described by Caballero et al. [38], who evaluated 35 patients aged between 3 and 16 years old aged with respiratory allergies and noted elevated total IgE in 77.2 % of the sample.
Atopic patients generally have specific IgE antibodies for several antigens, including fungi, as part of polysensitization. Allergic reactions and inhaled antigens have frequently been reported, although there has not been any proof that exposure to fungi transported by the air in outdoor environments plays any role in allergic rhinitis or other allergies of superior airways [3].
Among the 100 patients enrolled in the Pediatric Pneumology Program, 97 (97 %) demonstrated that IgE total concentrations were detected. 75 % were sensitized to Aspergillus, 87 % were sensitized to Penicillium; 46 % were sensitized to Neurospora and 45 % were sensitized to Fusarium.
A Mexican study involving pediatric patients with respiratory allergy showed that the most frequent allergens were Rhizopus, Aspergillus, Cladosporium and Candida. Total IgE was found to be high in 77.2 % of patients, and specific IgE was found to be high in 31.4 % [38].
In Malaysia, Goh et al. [39] found that 72.73 % of subjects had positive specific IgE against Penicillium. The result is similar to that of the patients in our study (87 %), can be explained by the use of ELISA in both studies and the high frequency of Penicillium in Brazil and Malaysia. According to Burge et al. [40], Penicillium is common in outdoor and indoor environments. Patients with asthma usually present positive responses for spores and other isolated Penicillium antigens.
Osorio et al. [41] found hypersensitivity to fungi, mainly in the genera Penicillium and Aspergillus, in 12 of 13 asthmatic schoolchildren in their study in the city of Recife. In a study conducted in Taiwan, Candida (72.1 %) was the most common sensitizing agent, followed by Aspergillus (51.2 %) and Penicillium (41.9 %) [42].
There was not statistic correlation with sex and local of residence and IgE total antibody concentrations or specific IgE to Aspergillus and Penicillium in this study. Considering that Aspergillus was the most frequently isolated antigen in this research, and it was also present in all areas of the city all year, it is reasonable to suppose that these fungus spores are naturally sensitizing. However, most of the subjects of this research lived in the central area of the city, which was where the fewest fungi were detected, possibly due to a larger concentration of buildings and homes.
Regarding age, there was statistical significant correlation between age and total IgE, but not between age and IgE anti-Aspergillus and anti-Penicillium. Chang et al. [42] found that the sensitization pattern revealed that fungal allergy was extremely common among children with respiratory allergic disease, especially in those <10 years of age. One possible reason is that younger children may be less efficient in clearing airway fungal spores, which results in higher and longer antigenic stimulation.
There was no correlation between total IgE and anti-Aspergillus IgE in the current research; however, this finding led us to suppose that elevated anti-Aspergillus IgE concentrations might have contributed to the elevation of serum total IgE.
Strenghts and limitations
This study with a large number of patients sensitized to Aspergillus and Penicillium, or less frequently to Fusarium and Neurospora spp., shows the importance of these sensitisations.
However, this study has some limitations. First, we have not accounted or discounted the possibility that the findings could be a result of indoor exposures on elevated IgE. In addition, the sample included only 100 children. The technique used to assess the levels of IgE ELISA was the index and not by Phamacia CAP unit system. Limitations in the statistical analysis. This is a cross-sectional study and there was no assessment of the levels of exposure to fungi and the development of allergic diseases. There was not a control group. Besides, the prick test was not conducted to confirm allergy to fungi of the study.
Conclusions
The genera of fungi identified in the present study were correlated with natural systems and could be useful in assessing the impact of environmental changes on the region studied.
In short, based on the data obtained in this study, the following aspects of the causal relationship between exposure and the symptoms of respiratory allergy may be noted. This study shows the importance of airborne fungi sensitizations in children with respiratory allergy. Combined diagnosis of total IgE test, specific IgE and clinical aspects is very useful for deciding specific therapies.
Finally, it was possible to conclude that the presence of specific IgE antibodies for the studied airborne fungi might be due to polysensitization because the fungi were present in all of the areas over the whole year in the city of São Luis-MA, Brazil, so the topic requires more study to understand fully airborne fungus respiratory allergies.
Abbreviations
- IgE:
-
E immunoglobulin
- LIF:
-
immunophysiology laboratory
- NIBA:
-
nucleum of basic and applied immunology
References
Shin SH, Lee YH. Airborne fungi induce nasal polyp epithelial cell activation and toll-like receptor expression. Int Arch Allergy Immunol. 2010;153(1):46–52.
Horner AA. Toll-like receptor ligands and atopy: a coin with at least two sides. J Allergy Clin Immunol. 2006;117(5):1133–40.
Bush RK, Faaaai MD, Portnoy JM, Saxon AMD, Terr AI, Wood RA. Environmental and occupational respiratory disorders. J Allergy Clin Immunol. 2006;117(2):326–33.
Thornton CR, Wills OE. Immunodetection of fungal and oomycete pathogens: established and emerging threats to human health, animal welfare and global food security. Crit Rev Microbiol. 2015;41(1):27–51.
Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Denning DW. Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology. 2007;153:1677–92.
Simon-Nobbe B, Denk U, Pöll V, Rid R, Breitenbach M. The spectrum of fungal allergy. Int Arch Allergy Immunol. 2008;145(1):58–86.
GINA. Global Burden of Asthma. 2010. Disponivel em: <http://w.w.w.ginasthma.com>. Acesso em: 12 jan 2011.
Halonen M, Stern DA, Wright AL, Taussig LM, Martinez FD. Alternaria as a major allergen for asthma in children raised in a desert environment. Am J Respir Crit Care Med. 1997;155:1356–61.
Nelson HS, Szefler SJ, Jacobs J, Huss K, Shapiro G, Sternberg AL. The relationships among environmental allergen sensitization, allergen exposure, pulmonary function, and bronchial hyperresponsiveness in the Childhood Asthma Management Program. J Allergy Clin Immunol. 1999;104:775–85.
Peat JK, Tovey E, Mellis CM, Leeder SR, Woolcock AJ. Importance of house dust mite and Alternaria allergens in childhood asthma: an epidemiological study in two climatic regions of Australia. Clin Exp Allergy. 1993;23:812–20.
Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113:227–34.
Neukirch C, Henry C, Leynaert B, Liard R, Bousquet J, Neukirch F. Is sensitization to Alternaria alternata a risk factor for severe asthma? a population-based study. J Allergy Clin Immunol. 1999;103:709–11.
Niedoszytko M, Chelminska M, Jassem E, Czestochowska E. Association between sensitization to Aureobasidium pullulans (Pullularia sp.) and severity of asthma. Ann Allergy Asthma Immunol. 2007;98:153–6.
Tillie L, Tonnel AB. Allergic bronchopulmonary aspergillosis. Allergy. 2005;60(8):1004–13.
Corry DB, Kheradmand F. Toward a comprehensive understanding of allergic lung disease. Trans Am Clin Climatol Assoc. 2009;120:33–48.
Feng M, Sun W, Cheng X. Seasonal dynamics and distribution of house mites in China. BioSci Trends. 2009;3(6):210–5.
Ho Shuk-Mei. environmental epigenetic of asthma—an update. J Allergy Clin Immunol. 2010;126(3):453–65.
Sharpe RA, Bearman N, Thornton CR, Husk K, Osborne NJ. Indoor fungal diversity and asthma: a meta-analysis and systematic review of risk factors. J Allergy Clin Immunol. 2015;135(1):110–22.
Sharpe RA, Cocq KL, Nikolaou V, Osborne NJ, Thornton CR. Identifying risk factors for exposure to culturable allergenic moulds in energy efficient homes by using highly specific monoclonal antibodies. Environ Res. 2016;144:32–42.
Tham R, Dharmage SC, Taylor PE, Katelaris CH, Vicendese D, Abramson MJ, Erbas B. Outdoor fungi and child asthma health service attendances. Pediatr Allergy Immunol. 2014;25(5):439–49.
Mezzari A, Perin C, Santos Junior SA, Bernd LAG, Gesu G, Airborne fungi and sensitization in atopic individuals in Porto Alegre, RS. Rev Assoc Med Bras. 2003;49(3):270–3.
Mendes SS, Mesquita JB, Ferrari SF, Assis JP. Airborne fungi in the city of Aracaju, Sergipe. Braz Biol Geral Exper. 2011;11:5–22.
Rios L. Estudos de Geografia do Maranhão. 1 ed. Graphis: São Luis; 2001. p. 106.
Bezerra GFB. Biodiversidade dos fungos do ar de São Luís—Maranhão [PhD Thesis]. São Luís: Rede Nordeste de Biotecnologia ponto focal Universidade Federal do Maranhão; 2011.
Menezes EA, Carvalho PG, Trindade ECPM, Madeira Sobrinho, Cunha FA, Castro FFM. Airborne fungi isolated from Fortaleza city, State of Ceará, Brazi. Rev Inst Med Trop. 2004;46(3):133–7.
Kato H, Sugita T, Ishibashi Y, Nishikawa A. Detection and quantification of specific ige antibodies against eight Malassezia Species in Sera of Patients with atopic dermatitis by using an enzyme-linked immunosorbent assay. Microbiol Immunol. 2006;50(11):851–6.
Onishi Y, Kuroda M, Yasueda H, Saito A, Sono-Koyama E, Tunasawa S, Hashida-Okado T, et al. Two-dimensional electrophoresis of Malassezia allergens for atopic dermatitis and isolation of Mal f 4 homologs with mitochondrial malate ehydrogenase. Eur J Biochem. 1999;261:148–54.
Kato H, Sugita T, Ishibashi Y, Nishikawa A. Evaluation of the levels of specificic IgE against Cryptococcus diffluens and Cryptococcus liquefaciens in patients with atopic dermatitis. Microbiol Immunol. 2007;51:945–50.
Alves R, Silva DA, Fernandes JF, Almeida KC, Ynoue LH, Bernardes CT, Moreira PF, et al. Humoral and cellular immune responses to Blomia tropicalis and concanavalin A-bidins fractions in atopic patients. Braz J Med Biol Res. 2008;41:773–81.
Soares CO. Princípios, padronização e validação de provas sorológicas. In: Madruga CR, Araújo FR, Soares CO. Imunodiagnóstico em Medicina Veterinária. Campo Grande: Embrapa Gado de Corte; p. 145–178.
Kasprzyk I. Aeromycology—main research fields of interest during the last 25 years. Ann Agric Environ Med. 2008;15:1–7.
Traid-Hoffman C, Jakob T, Behrendt H. Determinants of allergenicity. J Allergy Clin Immunol. 2009;123:558–66.
Rosas I, Calderón C, Ulloa M, Lacey J. Abundance of airborne Penicillium CFU in relation to urbanization in México City. Appl Environ Microbiol. 1993;59:2648–52.
Machado GMR. Fungos anemófilos de áreas do grande Recife. [Masters Dissertation] [Recife]: Universidade Federal de Pernambuco; 1979.
Oliveira MTB, Braz RFS, Ribeiro MAG. Airborne fungi isolated from Natal, State of Rio Grande do Norte, Brazil. Rev Microbiol (S. Paulo) 1993; 24:198–202.
Spalding SM, Wald V, Bernd LAG. IgE sérica total em atópicos e não atópicos na cidade de Porto Alegre. Rev Ass Med Bras. 2000;46(2):93–7.
Godinho R, Lanza M, Godinho A, Rodrigues A, Assiz TML. Frequency of positive skin tests for airborne allergic agents. Braz J Otorhinolaryngol. 2003;69(6):824–8.
Caballero RG, Nader O, Morfin MB. Correlation between positive skin tests to molds, total IgE, and specific IgE using ELISA and mold cultures from the environment of the pediatric allergy patient. Rev Alerg Mex. 2001;48(5):137–40.
Goh JCC, Juliana J, Malina O, Ngah ZU, Norhafizalena O. Prevalence of Penicillium specific IgE level and allergy symptoms among office workers in a selected company in Bangi, Malaysia. Trop Biomed. 2007;24:37–46.
Burge HA, Hoyer ME, Solomon WR. Quality control factors for Alternaria allergens. Mycotaxon. 1989;44(1):55–63.
Albuquerque Osório AC, Sellaro Lyra NR, Cavalcanti Sarinho ES. Hipersensibilidade a fungos em crianças asmáticas de uma comunidade do Recife, Pernambuco. Rev Bras Saude Matern Infant. 2006;6:245–51.
Chang FY, Lee JH, Yang YH, Yu HH, Wang LC, Lin YT, Chiang BL. Analysis of the serum levels of fungi-specific immunoglobulin E in patients with allergic diseases. Int Arch Allergy Immunol. 2011;154(1):49–56.
Authors’ contributions
GFBB and MDSBN participated in interpretation of data, drafted and critically revised the manuscript. GFBB and MDSBN contributed to study design, interpretation of data, and critically revised the manuscript. MACNS, RMS, WEMF, DMCH analyzed and assisted in interpretation of the data and assisted in drafting the manuscript. WEMF, GMCV, IGR and LZ contributed to interpretation of data and critically revised the manuscript. GFBB assisted in data acquisition and interpretation. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to express their gratitude: to Federal University of Maranhão (UFMA) for allowing the use of the NIBA and LIF facilities; to the University Hospital; and to FAPEMA, which funded the execution of the current research, process number 011/2006—PPSUS.
Competing interests
The authors declare that they have no competing interests.
Funding
Notice for support MS/CNPq/FAPEMA Nº 011/2006 - PPSUS. Foundation for Scientific Research and Technological Development of the State of Maranhão (FAPEMA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
de Barros Bezerra, G.F., Haidar, D.M.C., da Silva, M.A.C.N. et al. IgE serum concentration against airborne fungi in children with respiratory allergies. Allergy Asthma Clin Immunol 12, 18 (2016). https://doi.org/10.1186/s13223-016-0128-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13223-016-0128-y