Abstract
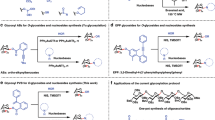
Here we present an overview of our work on the glycosylation of biologically relevant heterocycles.An array of stereochemically pure C-glycosylated dihydropyrimidine and dihydropyridine derivatives (artificial nucleosides) has been prepared. Our strategy involved the synthesis of suitably designed C-glycosylated reagents and their use as components in Biginelli and Hantzsch cyclocondensations. Molecular diversity has been explored within the collection on the basis of the nature and the number of sugar residues as well as their positions in the heterocyclic rings.
Similar content being viewed by others
References
Lebl, M., Parallel personal comments on 'classical' papers in combinatorial chemistry, J. Comb. Chem., 1 (1999) 3-24.
Fox, S., Farr-Jones, S. and Yund, M. A., High throughput screening for drug discovery: Continually transitioning into new technology, J. Biomol. Screening, 4 (1999) 183-186.
Wess, G., How to escape the bottleneck of medicinal chemistry, Drug Discov. Today, 7 (2002) 533-535.
Dean, P. M., Zanders, E. D. and Bailey, D. S., Industrialscale, genomics-based drug design and discovery, Trends Biotechnol., 19 (2001) 856-864.
Leach, A. R. and Hann, M. M., The in silico world of virtual libraries, Drug Discov. Today, 5 (2000) 326-336.
Golebiowski, A., Klopfenstein, S. R. and Portlock, D. E., Lead compounds discovered from libraries, Curr. Opin. Chem. Biol., 5 (2001) 273-284.
Waller, C. L., Recent advances in molecular diversity, Mol. Div., 5 (2000) 174-174.
Lipinski, C. A., Lombardo, F., Dominy, B. W. and Feeney, P.J., Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings, Adv. Drug. Delivery Rev., 46 (2001) 3-26.
Walters, W. P., Murcko, A. and Murcko, M. A., Recognizing molecules with drug-like properties, Curr. Opin. Chem. Biol., 3 (1999) 384-387.
Lipinski, C. A., Lombardo, F., Dominy, B. W. and Feeney, P. J., Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings, Adv. Drug. Delivery Rev., 23 (1997) 3-25.
Hann, M. M., Leach, A. R. and Harper, G., Molecular complexity and its impact on the probability of finding leads for drug discovery, Chem. Inf. Com., Sci., 41 (2001) 856-864.
Martin, E. J. and Critchlow, R. E., Beyond mere diversity: Tailoring combinatorial libraries for drug discovery, J.Comb. Chem., 1 (1999) 32-45.
Wess, G., Urmann, M. and Sickenberger, B., Medicinal chemistry: Challenges and opportunities, Angew. Chem. Int. Ed., 40 (2001) 3341-3350.
Li, A. P., Screening for human ADME/Tox drug properties in drug discovery, Drug Discov. Today, 6 (2001) 357-366.
Eddershaw, P. J., Beresford, A. P. and Bayliss, M. K., ADME/PK as part of a rational approach to drug discovery, Drug Discov. Today, 5 (2000) 409-414.
Breinbauer, R., Vetter, I. R. and Waldmann, H., From protein domains to drug candidates-Natural products as guiding principles in the design and synthesis of compound libraries, Angew. Chem. Int. Ed., 41 (2002) 2878-2890.
Varki, A., Cummings, R., Esko, J., Freeze, H., Hart, G. and Marth, J., Essential of Glycobiology, (eds.) Cold Spring Harbor Laboratory Press, New York, 1999.
Varki, A., Biological roles of oligosaccharides: all of the theories are correct, Glycobiology, 3 (1993) 97-130.
Barkley, A. and Arya, P., Combinatorial chemistry toward understanding the function(s) of carbohydrates and carbohydrate conjugates, Chem. Eur. J., 7 (2001) 555-563.
St Hilaire, P. M. and Meldal, M., Glycopeptide and oligosaccharide libraries, Angew. Chem. Int. Ed., 39 (2000) 1162-1179.
Wong, C.-H., Mimics of complex carbohydrates recognized by receptors, Acc. Chem. Res., 32 (1999) 376-385.
Sears, P. and Wong, C. H., Carbohydrate mimetics: A new strategy for tackling the problem of carbohydrate-mediated biological recognition, Angew. Chem. Int. Ed., 38 (1999) 2301-2324.
Evans, B. E., Rittle, K. E., Bock, M. G., DiPardo, R. M., Freidinger, R. M., Whitter, W. L., Lundell, G. F., Veber, D. F., Anderson, P. S. and Chang, R. S., Methods for drug discovery: development of potent, selective, orally effective cholecystokinin antagonists, J. Med. Chem., 31 (1988) 2235-2246.
Dolle, R. E., Comprehensive survey of combinatorial library synthesis: 2001, J. Comb. Chem., 4 (2002) 369-418.
Dolle, R. E., Comprehensive survey of combinatorial library synthesis: 2000, J. Comb. Chem., 3 (2001) 477-517.
Lin, P. S., Lee, C. L. and Sim, M. M., Synthesis of novel guanidinoglycoside: 2-glycosylamino 4,5-dihydro-6-pyrimidinone, J. Org. Chem., 66 (2001) 8243-8247.
Dondoni, A., Massi, A., Minghini, E. and Bertolasi, V., Dihydropyridine C-glycoconjugates by Hantzsch cyclocondensation. Synthesis of a C(6)-glycosylated nifedipine analogue, Helv. Chim. Acta, 85 (2002) 3331-3348.
Dondoni, A., Massi, A., Sabbatini, S. and Bertolasi, V., Three-component Biginelli cyclocondensation reaction using C-glycosylated substrates. Preparation of a collection of dihydropyrimidinone glycoconjugates and the synthesis of Cglycosylated monastrol analogues, J. Org. Chem., 67 (2002) 6979-6994.
Dondoni, A., Massi, A. and Minghini, E., Twoand three-component Hantzsch reaction using C-glycosylated reagents. Approach to the asymmetric synthesis of 1,4-diyhydropyridines, Synlett, 1 (2002) 89-92.
Dondoni, A., Massi, A. and Sabbatini, S., Towards the synthesis of C-glycosylated dihydropyrimidine libraries via the three-component Biginelli reaction. A novel approach to artificial nucleosides, Tetrahedron Lett., 42 (2001) 4495-4497.
Bienayme, H., Hulme, C., Oddon, G. and Schmitt, P., Maximizing synthetic efficiency: Multi-component transformations lead the way, Chem. Eur. J., 6 (2000) 3321-3329.
Ugi, I., Domling, A. and Werner, B., Since 1995 the new chemistry of multicomponent reactions and their libraries, including their heterocyclic chemistry, J. Heterocyclic Chem., 37 (2000) 647-658.
Lavilla, R., Recent developments in the chemistry of dihydropyridines, J. Chem. Soc., Perkin Trans. 1, 9 (2002) 1141-1156.
Stout, D. M. and Meyers, A. I., Recent advances in the chemistry of dihydropyridines, 82 (1982) 223-243.
For reviews see: (a) Kappe, C. O., Recent advances in the Biginelli dihydropyrimidine synthesis. New tricks from an old dog, Acc. Chem. Res., 33 (2000) 879-888.
Kappe, C. O., 100 Years of the Biginelli dihydropyrimidine synthesis, Tetrahedron, 49 (1993) 6937-6963.
Bossert, F. and Vater, W., US Pat. 3, 485, 1969.
Bossert, F. and Vater, W., Dihydropyridines, a new group of strongly effective coronary therapeutic agents, Naturwissenschaften, 58 (1971) 578.
For a survey on this topic with leading references, see: Kappe, C. O., Biologically active dihydropyrimidinones of the Biginelli-type: a literature survey, Eur. J. Med. Chem., 35 (2000) 1043-1052.
Klusa, V., Semenova, T. D., Medvinskaya, N. I. and Fast, A. E., Cerebrocrast (IOS-1212) normalizes disturbances of learning, attention, emotional behavior and brain biogenic amine levels in prenatally hypoxized rats, Latvijas Zinatnu Akademijas Vestis, 11/12 (1995) 156-161.
Mayer, T. U., Kapoor, T. M., Haggarty, S. J., King, R. W., Schreiber, S. L. and Mitchison, T. J., Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen, Science 286 (1999) 971-974.
Goldmann, S. and Stoltefuss, J., 1,4-dihydropyridines-effects of chirality and conformation on the calcium-antagonist and calcium agonist activities, Angew. Chem. Int. Ed., 30 (1991) 1559-1578.
Dondoni, A., Formylation of carbohydrates and the evolution of synthetic routes to artificial oligosaccharides and glycoconjugates, Pure App. Chem., 72 (2000) 1577-1588.
Dondoni, A. and Schernnann, M. C., Thiazole-based synthesis of formyl C-glycosides, J. Org. Chem., 59 (1994) 6404-6412.
Postema, M. H. D., C-Glycoside Synthesis, CRC Press, Boca Raton, 1995, pp. 303-342.
Chu, C. K. and Baker, D. C., Nucleosides and Nucleotides as Antitumor and Antiviral Agents, (eds.) Plenum Press, New York, 1993.
Hu, E. H., Sidler, D. R. and Dolling, U. H., Unprecedented catalytic three component one-pot condensation reaction: An efficient synthesis of 5-alkoxycarbonyl-4-aryl-3,4-dihydropyrimidin-2(1H)-ones, J. Org. Chem., 63 (1998) 3454-3457.
Ma, Y., Qian, C. T., Wang, L. M. and Yang, M., Lanthanide triflate catalyzed Biginelli reaction. One-pot synthesis of dihydropyrimidinones under solvent-free conditions, J. Org. Chem., 65 (2000) 3864-3868.
Uray, G., Verdino, P., Belaj, F., Kappe, C. O. and Fabian, W. M. F., Absolute configuration in 4-alkyl-and 4-aryl-3,4-dihydro-2(1H)-pyrimidones: A combined theoretical and experimental investigation, J. Org. Chem., 66 (2001) 6685-6694.
Kappe, C. O., Shishkin, O. V., Uray, G. and Verdino, P., Synthesis and reactions of Biginelli compounds, part 19-X-ray structure, conformational analysis, enantioseparation, and determination of absolute configuration of the mitotic kinesin Eg5 inhibitor monastrol, Tetrahedron, 56 (2000) 1859-1862.
Del Poeta, M., Schell, W. A., Dykstra, C. C., Jones, S. K., Tidwell, R. R., Kumar, A., Boykin, D. W. and Perfect, J. R., In vitro antifungal activities of a series of dication-substituted carbazoles, furans, and benzimidazoles, Antimicrob. Agents Chemother., 42 (1998) 2503-2510.
Sasho, S., Obase, H., Ichikawa, S., Kitazawa, T., Nonaka, H., Yoshizaki, R., Ishii, A. and Shuto, K., Synthesis of 2-imidazolidinylidenepropanedinitrile derivatives as stimulators of gastrointestinal motility 1, J. Med. Chem., 36 (1993) 572-579.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dondoni, A., Massi, A. Decoration of dihydropyrimidine and dihydropyridine scaffolds with sugars via Biginelli and Hantzsch multicomponent reactions: An efficient entry to a collection of artificial nucleosides. Mol Divers 6, 261–270 (2003). https://doi.org/10.1023/B:MODI.0000006806.91483.a3
Issue Date:
DOI: https://doi.org/10.1023/B:MODI.0000006806.91483.a3