Abstract
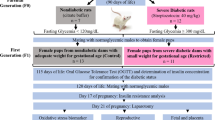
The aim of this study was to determine the possible fetal effects of interaction between maternal diabetes and acute doses of alcohol. Pregnant TO mice were made diabetic by a single injection of streptozotocin (STZ) on gestation day (GD) 2. Single dose of 0.003 or 0.03 ml/g body weight of fresh ethanol (25% v/v of absolute alcohol in normal saline) was injected into groups of diabetic and nondiabetic animals on GD 7 or 8. One group of diabetic animals had a daily dose of 6–8 IU of insulin subcutaneously. Fetuses were collected on GD 18. There was a significant increase in the incidence of implantation failure in the diabetes plus ethanol groups and insulin control group. Ethanol injection on GD 7 accentuated diabetes-related embryonic resorption and intrauterine growth retardation (IUGR). This effect was less marked in the diabetic group treated with ethanol on GD 8. Diabetes alone produced a greater incidence of IUGR than ethanol alone. Midfacial hypoplasia and minor anomalies were found more frequently in the combination treatment groups. Holoprosencephaly and thymus hypoplasia observed in diabetic groups were found to be reduced in frequency in the diabetes plus ethanol groups, suggesting an antagonistic type of ethanol--diabetes interaction, stage-dependently. Since severely malformed embryos are known to be resorbed/killed in utero in mice, this reduction might reflect the magnitude of early death of severely malformed embryos. These data suggest that the interaction effects are possibly related to alterations in fundamental developmental processes of early embryos. (Mol Cell Biochem 261: 43–56, 2004)
Similar content being viewed by others
References
von Kries R, Kimmerle R, Schmidt JE, Hachmeister A, Bohm O, Wolf HG: Pregnancy outcomes in mothers with pregestational diabetes: A population-based study in North Rhine (Germany) from 1988 to 1993. Eur J Pediatr 156: 963–967, 1997
Greene MF: Spontaneous abortions and major malformations in women with diabetes mellitus. Semin Reprod Endocrinol 17: 127–136, 1999
Manderson JG, Mullan B, Patterson CC, Hadden DR, Traub AI, McCance DR: Cardiovascular and metabolic abnormalities in the off-spring of diabetic pregnancy. Diabetologia 45: 991–996, 2002
Garner P: Type I diabetes mellitus and pregnancy. Lancet 346: 157–161, 1995
Kitzmiller JL, Buchanan TA, Kjos S, Combs CA, Ratner RE: Preconception care of diabetes, congenital malformations, and spontaneous abortions. Diabetes Care 19: 514–541, 1996
Sheffield JS, Butler-Koster EL, Casey BM, McIntire DD, Leveno KJ: Maternal diabetes mellitus and infant malformations. Obstet Gynecol 100(5, pt 1): 925–930, 2002
Pedersen J: The Pregnant Diabetic and Her Newborn. Problems and Management, 2nd edn. Munsgaard, Copenhagen, 1977, pp 191–197
Cundy T, Gamble G, Townsend K, Henley PG, Mac Pherson P, Roberts AB: Perinatal mortality in Type diabetes mellitus. Diabet Med 17: 33–39, 2000
Buchanan TA, Kitzmiller JL: Metabolic interactions of diabetes and pregnancy. Annu Rev Med 45: 245–260, 1994
Lofffredo C, Wilson PD, Ferencz C: Maternal diabetes: An Independent risk factor for major cardiovascular malformations with increased mortality of affected infants. Teratology 64: 98–106, 2001
Reece EA, Homko CJ: Why do diabetic women deliver malformed infants? Clin Obstet Gynecol 43: 32–45, 2000
Buchanan TA, Denno KM, Sipos GF, Sadler TW: Diabetic teratogenesis. In vitro evidence for a multifactorial etiology with little contribution from glucose per se. Diabetes 43: 656–660, 1994
Styrud J, Thunberg L, Nybacka O, Eriksson UJ: Correlations between maternal metabolism and deranged development in the offspring of normal and diabetic rats. Pediatr Res 37: 343–353, 1995
Gunton JE, McElduff A, Sulway M, Stiel J, Kelso I, Boyce S, Fulcher G, Robinson B, Clifton-Bligh P, Wilmshurst E: Outcome of pregnancies complicated by pre-gestational diabetes mellitus. Aust N Z J Obstet Gynaecol 40: 38–43, 2000
Centers for Disease Control and Prevention: Alcohol consumption among pregnant and childbearing-aged women—United States, 1991 and 1995. MMWR 46(16): 345–350, 1997
Ajani UA, Henneckens CH, Spelberg A, Manson JE: Alcohol consumption and risk of type-2 diabetes mellitus among US male physicians. Arch Intern Med 160: 1025–1030, 2000
Conigrave KM, Hu BF, Camargo CA Jr, Stampfer MJ, Willett WC, Rimm EB: A prospective study of drinking patterns in relation to risk of type-2 diabetes among men. Diabetes 50: 2390–2395, 2001
Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC: Diet, lifestyle, and the risk of type-2 diabetes mellitus in women. N Engl J Med 346: 790–797, 2001
Hodge AM, Dowse GK, Collins VR, Zimmel PZ: Abnormal glucose tolerance and alcohol consumption in the populations at high risk of non-insulin dependent diabetes mellitus. Am J Epidemiol 137: 178–189, 1993
Todoroki J, Shinchi K, Kono S, Imanishi K: Lifestyle and glucose tolerance: A cross sectional study of Japanese men. Ann Epidemiol 4: 363–368, 1994
Holbrook TL, Barett-Connor E Wingard DL: A prospective study of alcohol use and non-insulin dependent diabetes mellitus. Am J Epidemiol 132: 902–909, 1990
Balkau B, Randrianjohany A, Papoz L, Eschwege E: A prospective study of alcohol use and non-insulin dependent diabetes mellitus. Am J Epidemiol 134: 1469–1470, 1991
Majewski F, Nothjunge J, Bierich JR: Alcohol embryopathy and diabetic fetopathy in the same newborn. Helv Paediatr Acta 34: 135–139, 1979
Lemoine P, Harousseau H, Borteryu JP, Menuet JC: Les enfants de parents alcoholiques: Anomalies observe a propos des 127 cas. Quest Med 21: 476–482, 1968
Padmanabhan R, Wasfi IA, Craigmyle MB: Effect of pre-treatment with aspirin on the neural tube defects induced by maternal exposure to alcohol in the TO mouse fetuses. Drug Alcohol Depend 36: 175–186, 1994
Floyd RL, Decoufle P, Hungerford DW: Alcohol use prior to pregnancy recognition. Am J Prev Med 17: 101–107, 1999
Hannigan JH, Armant DR: Alcohol in pregnancy and neonatal outcome. Semin Neonatol 5: 243–254, 2000
Li TK, Yin SJ, Crabb DW, O'Connor S, Ramchandani VA: Genetic and environmental influences on alcohol metabolism in humans. Alcohol Clin Environ Res 25: 136–144, 2001
Padmanabhan R, Hameed MS, Sugathan TN: Effects of acute doses of ethanol on pre and postnatal development in the mouse. Drug Alcohol Depend 14: 197–206, 1984
Padmanabhan R, Muawad WMRA: Exencephaly and axial skeletal dysmorphogenesis induced by acute doses of ethanol in mouse fetuses. Drug Alcohol Depend 16: 215–227, 1985
Zajac CS, Abel EL: Animal models of prenatal alcohol exposure. Int J Epidemiol 21(suppl 1): S24–S32, 1992
Randall CL Alcohol and pregnancy: Highlights from three decades of research. J Stud Alcohol 62: 554–561, 2001
Padmanabhan R, Al-Zuhair AGH: Congenital malformations and intrauterine growth retardation in streptozotocin-induced diabetes during gestation in the rat. Reprod Toxicol 1: 117–125, 1987
Padmanabhan R, Shafiullah: Intrauterine growth retardation in experimental diabetes. Possible role of the placenta. Arch Physiol Biochem 109: 260–271, 2001
Wilson JG: Environment and Birth Defects. Academic Press, New York, 1973
Sterz H, Lehmann H: A critical comparison of the free hand razor blade dissection method according to Wilson with an in situ sectioning method for rat fetuses. Terat Carcin Mutagen 5: 347–354, 1985
Padmanabhan R, Ahmad I: Sodium valproate augments spontaneous neural tube defects and axial skeletal malformations in TO mouse fetuses. Reprod Toxicol 10: 345–363, 1996
Padmanabhan R: Retinoic acid-induced caudal regression syndrome the mouse fetus. Reprod Toxicol 12: 139–151, 1998
Jones KL, Smith DW: Recognition of the fetal alcohol syndrome early infancy. Lancet 2: 999–1001, 1973
Jones KL, Smith DW, Ulleland CN, Streissguth AP: Pattern of malformation in offspring of chronic alcoholic mothers. Lancet 1: 1267–1271, 1973
Hanson JW, Jones KL, Smith DW: Fetal alcohol syndrome, experience with 41 patients. JAMA 235: 1458–1460, 1976
Streissguth AP, Landesman-Dwyer S, Martin J, Smith DW: Teratogenic effects of alcohol in humans and animals. Science 209: 353–361, 1980
Becker HC, Diaz-Grandos JL, Randall CL: Teratogenic actions ethanol in the mouse: A mini review. Pharmacol Biochem Behav 55: 501–513, 1996
Pedersen JF, Molsted-Pederson L: Early fetal growth delay detected by ultrasound marks increased risk of congenital malformation in diabetic pregnancy. Br Med J 283: 269–271, 1981
Eriksson UJ, Lewis NJ, Freinkel N: Growth retardation during early organogenesis in embryos of experimentally diabetic rats. Diabetes 33: 281–284, 1984
Wilson GN, Howe M, Stover JM: Delayed developmental sequences rodent diabetic embryopathy. Pediatr Res 19: 1337–1340, 1985
McCarter RJ, Kessler II, Comstock GW: Is diabetes mellitus a teratogen or a coteratogen? Am J Epidemiol 125: 195–205, 1987
Eriksson UJ, Dahlstrom E, Hellerstrom C: Diabetes in pregnancy. Skeletal malformations in the offspring of diabetic rats after intermittent withdrawal of insulin in early gestation. Diabetes 32: 1141–1145, 1983
Rosen P, Nawroth PP, King G, Moller W, Tritschler HJ, Packer L: The role of oxidative stress in the onset and progression of diabetes and its complications: A summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev 17: 189–212, 2001
Chen SY, Sulik KK: Iron-mediated free radical injury in ethanol-exposed mouse neural crest cells. J Pharmacol Exp Ther 294: 134–140, 2000
Dunty WC Jr, Zucker RM, Sulik KK: Hindbrain and cranial nerve dysmorphogenesis result from acute maternal ethanol administration. Dev Neurosci 24(4): 328–342, 2002
Phelan SA, Ito M, Loeken MR: Neural tube defects in embryos of diabetic mice: Role of the Pax-3 gene and apoptosis. Diabetes 46: 1189–1197, 1997
Chan BWH, Chan KS, Koide T, Yeung SM, Leung MB, Copp AJ, Loeken MR, Shiroishi T, Shum AS: Maternal diabetes increases the risk of caudal regression caused by retinoic acid. Diabetes 51: 2811–2816, 2002
Davis WI, Crawford LA, Cooper OJ, Farmer GR, Thomas DL, Freeman BL: Ethanol induces the generation of reactive free radicals by neural crest cells in vitro. J Craniofac Genet Dev Biol 10: 277–293, 1990
Simán CM, Gettenberger-De, Groot AC, Wisse B, Eriksson UJ: Malformation in offspring of diabetic rats: Morphmetric analysis of neural crest derived organs and effects of maternal vitamin E treatment. Teratology 61: 355–367, 2000
Cohen-Kerem R, Koren G: Antioxidants and fetal protection against ethanol teratogenicity, I: Review of the experimental data and implications to humans. Neurotoxicol Teratol 25: 1–9, 2003
Copp AJ, Crolla JA, Brook FA: Prevention of spinal neural tube defects in the mouse embryo by growth retardation during neurulation. Development 104: 297–303, 1988
Banta JV, Nichols O: Sacral agenesis. J Bone Joint Surg Am 51: 693–703, 1969
Passarge E, Lenz W: Syndrome of caudal regression in infants of diabetic mothers: Observations of further cases. Pediatrics 37: 672–675, 1966
Cohen MM Jr, Shiota K: Teratogenesis of holoprosencephaly. Am Med Genet 109: 1–15, 2002
Roessler E, Muenke M: Howa Hedgehog might see holoprosencephaly. Hum Mol Genet 12(suppl 1): R15–R25, 2003
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Padmanabhan, R., Shafiullah, M. Effect of maternal diabetes and ethanol interactions on embryo development in the mouse. Mol Cell Biochem 261, 43–56 (2004). https://doi.org/10.1023/B:MCBI.0000028736.00532.1e
Issue Date:
DOI: https://doi.org/10.1023/B:MCBI.0000028736.00532.1e