Abstract
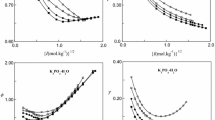
The mixture {yNaCl + (1 − y)CaCl2}(aq) has been studied with the hygrometric method at the temperature 25°C. The water activities were measured at total molalities from 0.25 mol-kg−1 to near saturation for different ionic-strength fractions y of NaCl with y = 0.33, 0.50, and 0.67. The obtained data allow the calculation of osmotic coefficients. The experimental results are compared with the predictions of the Zdanovskii–Stokes–Robinson (ZSR), Kusik and Meissner (KM), Robinson and Stokes (RS), Lietzke and Stoughton (LS II), Reilly–Wood–Robinson (RWR), and Pitzer models. From the measured osmotic coefficients, the Pitzer ionic mixing parameters θNa Ca and ψNa Ca Cl are determined and are used to predict the solute activity coefficients in the mixtures. The excess Gibbs energy is also determined. These results are compared with those given in the literature.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Guendouzi, M.E., Azougen, R., Dinane, A. et al. Hygrometric Determination of the Water Activities of the Aqueous Solutions of the Mixed Electrolyte NaCl–CaCl2–H2O at 25°C. Journal of Solution Chemistry 33, 941–955 (2004). https://doi.org/10.1023/B:JOSL.0000048046.88369.88
Issue Date:
DOI: https://doi.org/10.1023/B:JOSL.0000048046.88369.88