Abstract
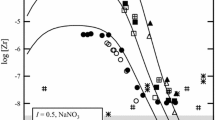
The stability constants of zirconium(IV) hydrolysis species have been measured at 15, 25, and 35 °C [in 1.0 mol-dm−3 (H,Na)ClO4] using both potentiometry and solvent extraction. In addition, the solubility of [Zr(OH)4(am)] has been investigated in a 1 mol-dm−3 (Na,H)(ClO4,OH) medium at 25 °C over a wide range of −log [H+] (0-15). The results indicate the presence of the monomeric species Zr(OH)3+, Zr(OH)2 2+, Zr(OH)3 +, and Zr(OH)4 0(aq) as well as the polymeric species Zr4(OH)8 8+ and Zr2(OH)6 2+. The solvent extraction measurements required the use of acetylacetone. As such, the stability constants of zirconium(IV) with acetylacetone were also measured using solvent extraction. All stability constants were found to be linear functions of the reciprocal of temperature (in kelvin) indicating that Δ H o and Δ S o are both independent of temperature (over the temperature range examined in the study). The results of the solubility experiments have shown four distinctly different solubility regions. In strongly acidic solutions, the solubility is controlled by the formation of polynuclear hydrolysis species in solution whereas in less acidic solution the formation of mononuclear hydrolysis species becomes dominant. The largest portion of the solubility curve is controlled by equilibrium with aqueous Zr(OH)4 0(aq) where the solubility is independent of the proton concentration. In alkaline solutions, the solubility increases due to formation of the zirconate ion. The middle region was used to determine the solubility constant (log *K s10) of Zr(OH)4(s). From the data in the alkaline region, a value of the stability of the zirconate ion has been determined. This is the first time that the possible evidence for the zirconate ion has been identified in aqueous solution that has previously been found only in the solid phase.
Similar content being viewed by others
References
C. F. Baes and R. E. Mesmer, The Hydrolysis of Cations, 2nd edn. Kreiger, New York, (1986).
M. A. Pouchon, E. Curti, C. Degueldre, and L. Tobler, Progr. Nucl. Energy 38, 443(2001).
W. H. McVey, United States Atomic Energy Commission Rep., HW-21487, 1951.
A. J. Zielen and R. E. Connick, J. Amer. Chem. Soc. 78, 5785(1956).
G. M. Muha and P. A. Vaughan, J. Chem. Phys. 33, 194(1960).
S. Tribalat and L. Schriver, Bull. Soc. Chim. Fr. 9, 2012(1975).
A. J. Veyland, L. Dupont, J.-C. Pierrand, J. Rimbault, and M. Aplincourt, Eur. J. Inorg. Chem. p. 1765(1998).
I. A. Sheka and Ts. V. Pevzner, Russ. J. Inorg. Chem. 5, 1119(1960).
S. U. Aja, S. A. Wood, and A. E. Williams-Jones, Appl. Geochem. 10, 603(1995).
J. H. Adair, H. G. Krarup, S. Venigalla, and T. Tsukada, Mat. Res. Soc. Symp. Proc. 432, 101(1997).
A. S. Solovkin and A. I. Ivantsov, Russ. J. Inorg. Chem. 11, 1013(1966).
V. A. Nazarenko and O. V. Mandzhgaladze, Russ. J. Inorg. Chem. 14, 639(1969).
V. M. Peshkova, N. V. Mel'chakova, and S. G. Zhemchuzhin, Russ. J. Inorg. Chem. 6, 630(1961).
A. S. Solovkin, Z. N. Tsvetkova, and A. I. Ivantsov, Russ. J. Inorg. Chem. 12, 326(1967).
P. N. Kovalenko and K. N. Bagdasarov, Russ. J. Inorg. Chem. 6, 272(1961).
H. Bilinski and M. Branica, Croat. Chem. Acta 38, 263(1966).
H. Bilinski, M. Branica, and L. G. Sillén, Acta Chem. Scand. 20, 853(1966).
C. Ekberg, P. L. Brown, J. Comarmond, and Y. Albinsson, Mat. Res. Soc. Symp. Proc., Sci. Basis Nucl. Waste Manage. 663, 1091(2001).
P. L. Brown, M. E. Shying, and R. N. Sylva, J. Chem. Soc., Dalton Trans. p. 2149(1987).
P. L. Brown, Ph. D. dissertation, University of Wollongong, Wollongong, Australia, 1984.
C. Ekberg, Y. Albinsson, M. J. Comarmond, and P. L. Brown, J. Solution Chem. 29, 63(2000).
G. H. Khoe, P. L. Brown, R. N. Sylva, and R. G. Robins, J. Chem. Soc. Dalton Trans. p. 1901(1986).
P. L. Brown and R. J. Bowdler, Australian Nuclear Science and Technology Organisation Rep., ANSTO/C120, 1990.
J. Rydberg, Acta Chem. Scand. 23, 647(1969).
H. Reinhardt and J. Rydberg, Acta Chem. Scand. 23, 2773(1969).
C. Andersson, S. O. Andersson, J.-O. Liljenzin, H. Reinhardt, and J. Rydberg, Acta Chem. Scand. 23, 2781(1969).
H. Johansson and J. Rydberg, Acta Chem. Scand. 23, 2797(1969).
Y. Albinsson, L. E. Ohlsson, H. Persson, and J. Rydberg, Appl. Radiat. Isot. 39, 113(1988).
C. Ekberg, P. L. Brown, A. Ödegaard-Jensen, D. M. Hill, and A. Zawadzki, Anal. Bioanal. Chem. 374, 1330(2002).
A. Sabatini, A. Vacca and P. Gans, Talanta 21, 53(1974).
P. Gans, A. Sabatini, and A. Vacca, Inorg. Chim. Acta 18, 237(1976).
R. N. Sylva and M. R. Davidson, J. Chem. Soc. Dalton Trans. p. 232(1979).
J. Bjerrum, Ph. D. dissertation, Haase and Son, Copenhagen, Denmark, 1941.
C. Ekberg, G. Meinrath, A. Landgren, and J.-O. Liljenzin, in preparation.
I. Grenthe, J. Fuger, R. J. M. Konings, R. J. Lemire, A. B. Muller, C. Nguyen-Trung, and H. Wanner, Chemical Thermodynamics of Uranium, (North-Holland, Amsterdam, (1992).
Yu. P. Davydov and V. N. Zabrodskii, Vestsi Akad. Navuk BSSR, Ser. Khim. Navuk, 2, 3(1987).
B. Norén, Acta Chem. Scand. 27, 1369(1973).
V. I. Paramonova and A. N. Sergeev, Zh. Neorg. Khim. 3, 215(1958).
E. Curti, Paul Scherrer Institut Rep. TM-44-01-01, 2001.
R. J. Lemire, J. Fuger, H. Nitsche, P. Potter, M. H. Rand, J. Rydberg, K. Spahiu, J. C. Sullivan, W. J. Ullman, P. Vitorge, and H. Wanner, Chemical Thermodynamics of Neptunium and Plutonium (North-Holland, Amsterdam, 2001).
P. G. Daniele, C. Rigano, S. Sammartano, and V. Zelano, Talanta, 41, 1577(1994).
D. K. Nordstrom, L. N. Plummer, D. Langmuir, E. Busenburg, H. M. May, B. F. Jones, andD. L. Parkhurst, in Chemical Modelling of Aqueous Systems II, American Chemical Society Symposium Series No. 416, D. C. Melchor and R. L. Bassett, Eds. (American Chemical Society, Washington, DC, 1990), p. 398.
A. J. Zielen, University of California, Radiation Laboratory, Rep. UCRL-2268, 1953.
P. L. Brown, J. Ellis, and R. N. Sylva, J. Chem. Soc. Dalton Trans. p. 31(1983).
I. M. Korenman, F. R. Sheyanova, and Z. M. Gureva, Russ. J. Inorg. Chem. 11, 1485(1966).
J. Rydberg, Acta Chem. Scand. 14, 157(1960).
J.-O. Liljenzin and J. Stary, J. Inorg. Nucl. Chem. 32, 1357(1970).
M. A. Pouchon, E. Curti, C. Degueldre, and L. Tobler, Progr. Nucl. Energy 38, 443(2001).
F. A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry, 3rd edn. (Wiley, New York, 1972).
J. Bruno, D. Ferri, I. Grenthe, and F. Salvatore Acta Chem. Scand. 40, 428(1986).
D. Rai, J. L. Swanson, and J. L. Ryan, Radiochim. Acta 42, 35(1987).
D. Rai, Radiochim. Acta 35, 97(1984).
J. I. Kim, B. Kanellakopoulos, M. Mang, G. Herrmann, and H. Trautmann, Radiochim. Acta 48, 145(1989).
P. N. Kovalenko and K. N. Bagdasarov, Russ. J. Inorg. Chem. 6, 272(1961).
A. A. Slobodov, A. V. Kristskii, V. I. Zarembo, and L. V. Puchkov, Zh. Prikl. Khim. 65, 1031(1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ekberg, C., Källvenius, G., Albinsson, Y. et al. Studies on the Hydrolytic Behavior of Zirconium(IV). Journal of Solution Chemistry 33, 47–79 (2004). https://doi.org/10.1023/B:JOSL.0000026645.41309.d3
Issue Date:
DOI: https://doi.org/10.1023/B:JOSL.0000026645.41309.d3