Abstract
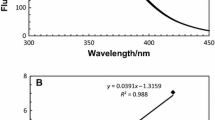
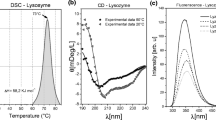
The secondary structure of bovine β-casein was characterized using circular dichroism (CD) and FTIR spectroscopies under physiologically relevant conditions. Analytical ultracentrifugation technique was used to follow the highly temperature, pH and concentration dependent self-association behavior. CD measurements provide convincing evidence for short segments of polyproline II-like structures in β-casein in addition to a wide range of secondary structure elements, such as 10–20% α-helix, ∼30% turns, 32–35% extended sheet. Results obtained at extreme pH (10.5) revealed structural destabilization in the monomeric form of the protein. At least four distinct structural transitions at 10, 33, 40 and 78°C were observed at pH 6.75 by CD analysis, compared to only two transitions, 26 and 40°C, at pH 10.5. Calculations from analytical ultracentrifugation suggest that the transitions at lower temperature (≤30°C) occur primarily in the monomer. It is hypothesized that the transition at 10°C and neutral pH may represent a general conformational change or cold denaturation. Those middle ranged transitions, i.e. 33 and 40°C are more likely the reflection of hydrophobic changes in the core of β-casein. As β-casein undergoes self-association and increases in size, the transition at higher temperature (78°C) is perhaps caused by the apparent conformational change within the micelle-like polymers. It has been shown that β-casein binds the hydrophobic fluorescent probe ANS with high affinity in much similar fashion to molten globular proteins. The effect of urea denaturation on the bound complex effectively supports this observation.
Similar content being viewed by others
REFERENCES
Adzhubei, A. A., and Sternberg, M. J. E. (1993). J. Mol. Biol. 229: 472–493.
Alaimo, M. H., Wickham, E. D., and Farrell, H. M., Jr. (1999). Biochim. Biophys. Acta 1431: 395–409.
Andrews, A. L., Atkinson, D., Evans, M. T. A., Finer, E. G., Green, J. P., Phillips, M. C., et al. (1979). Biopolymers 18: 1105–1121.
Bailey, R. W., Dunker, A. K., Brown, C. J., Garner, E. C., and Griswold, M. D. (2001). Biochemistry 40: 11828–11840.
Buchheim, W., and Schmidt, D. G. (1979). J. Dairy Res. 46: 277–280.
Byler, D. M., Farrell, H. M., Jr., and Susi, H. (1988). J. Dairy Sci. 71: 2622–2629.
Caessens, P. W., De Jongh, H. H., Norde, W., and Gruppen, H. (1999). Biochim. Biophys. Acta 1430: 73–83.
Chatchatee, P., Jarvinen, K. M., Bardina, L., Vila, L., Beyer, K., and Sampson, H. A. (2001). Clin. Exp. Allergy 31: 1256–1262.
Chou, P. Y., and Fasman, G. D. (1978). Adv. Enzymol. Relat. Areas Mol. Biol. 47: 45–178.
Cicuta, P., and Hopkinson, I. (2001). J. Chem. Phys. 114: 8659–8670.
Creamer, L. K. (1980). Arch. Biochem. Biophys. 199: 172–178.
Creamer, L. K., Richardson, T., and Parry, D. A. (1981). Arch. Biochem. Biophys. 211: 689–696.
Curley, D. M., Kumosinski, T. F., Unruh, J. J., and Farrell, H. M., Jr. (1998). J. Dairy Sci. 81: 3154–3162.
Dickinson, E., Semenova, M. G., Belyakova, L. E., Antipova, A. S., Il'in, M. M., Tsapkina, E. N., et al. (2001). J. Colloid Interface Sci. 239: 87–97.
Dukor, R. K., and Keiderling, T. A. (1991). Biopolymers 31: 1747–1761.
Dunker, A. K., and Obradovi, Z. (2001). Nat. Biotechnol. 19: 805–806.
Dyson, H. J., and Wright, P. E. (2002). Adv Protein Chem 62: 311–340.
Eigel, W. N., Butler, J. E., Ernstrom, C. A., Farrell, H. M., Jr., Harwalkar, V. R., Jenness, R., et al. (1984). J. Dairy Sci. 67: 1599–1631.
Farrell, H. M., Jr. (1999). In Encyclopedia of Reproduction, Volume 3 (Knobil, E. and Neill, J. D., ed.), Academic Press, Inc., San Diego, CA, pp. 256–263.
Farrell, H. M., Jr., Kumosinski, T. F., Pulaski, P., and Thompson, M. P. (1988). Arch. Biochem. Biophys. 265: 146–158.
Farrell, H. M., Jr., Deeney, J. T., Hild, E. K., and Kumosinski, T. F. (1990). J. Biol. Chem. 265: 17637–17643.
Farrell, H. M., Jr., Qi, P. X., Wickham, E. D., and Unruh, J. J. (2002). J. Protein Chem. 21: 307–321.
Farrell, H. M., Jr., Wickham, E. D., Unruh, J. J., Qi, P. X., and Hoagland, P. D. (2001). Food Hydrocolloids 15: 341–354.
Farrell, H. M., Jr., Qi, P. X., Brown, E. M., Cooke, P. H., Tunick, M. H., Wickham, E. D., et al. (2002). J. Dairy Sci. 85: 459–471.
Fragneto, G., Thomas, R. K., Rennie, A. R., and Penfold, J. (1995). Science 267: 657–660.
Garnier, J. (1966). J. Mol. Biol. 19: 586–590.
Graham, E. R. B., Malcolm, G. N., and McKenzie, H. A. (1984). Int. J. Biol. Macromol. 6: 155–161.
Greenberg, R., Groves, M. L., and Dower, H. J. (1984). J. Biol. Chem. 259: 5132–5138.
Grusby, M. J., Mitchell, S. C., Nabavi, N., and Glimcher, L. H. (1990). Proc. Natl. Acad. Sci. USA 87: 6897–6901.
Herskovits, T. T. (1966). Biochemistry 5: 1018–1026.
Holt, C., and Sawyer, L. (1993). J. Chem. Soc. Faraday Trans. 89: 2683–2692.
Javor, G. T., Sood, S. M., Chang, P., and Slattery, C. W. (1991). Arch. Biochem. Biophys. 289: 39–46.
Kawahara, K., and Tanford, C. (1966). J. Biol. Chem. 241: 3228–3232.
Kay, B. K., Williamson, M. P., and Sudol, M. (2000). FASEB J. 14: 231–241.
Kendrick, B. S., Dong, A., Allison, S. D., Manning, M. C., and Carpenter, J. F. (1996). J. Pharm. Sci. 85: 155–158.
Krimm, S., and Bandekar, J. (1986). Adv. Protein Chem. 38: 181–364.
Kumosinski, T. F., and Unruh, J. J. (1996). Talanta 43: 199–219.
Kumosinski, T. F., Brown, E. M., and Farrell, H. M., Jr. (1993). J. Dairy Sci. 76: 931–945.
Makarov, A. A., Lobachov, V. M., Adzhubei, I. A., and Esipova, N. G. (1992). FEBS Lett. 306: 63–65.
McLachlan, C. N. (2001). Med. Hypotheses 56: 262–272.
Noelken, M., and Reibstein, M. (1968). Arch. Biochem. Biophys. 123: 397–402.
O'Connell, J. E., Grinberg, V. Y., and de Kruif, C. G. (2003). J. Colloid Interface Sci. 258: 33–39.
Park, S. H., Shalongo, W., and Stellwagen, E. (1997). Protein Sci. 6: 1694–1700.
Payens, T. A. J., and van Markwijk, B. W. (1963). Biochim. Biophys. Acta 71: 517–530.
Pearce, K. N. (1975). Eur. J. Biochem. 58: 23–29.
Plaxco, K. W., and Gross, M. (2001). Nat Struct Biol 8: 659–60.
Provencher, S. W., and Glockner, J. (1981). Biochemstry 20: 33–37.
Ptitsyn, O. B. (1995). Adv. Protein Chem. 47: 83–229.
Qi, P. X., Wickham, E. D., Piotrowski, E. G., Fagerquist, C. K., and Farrell, H. M., Jr. (2004). Submitted for publication to Arch. Bichem. Biophys.
Rucker, A. L., and Creamer, T. P. (2002). Protein Sci. 11: 980–985.
Rusling, J. F., and Kumosinski, T. F. (1996). In Nonlinear computer modeling of chemical and biochemical data, Academic Press, San Diego, CA.
Schellman, J. A. (1990). Biophys. Chem. 37: 121–140.
Schmidt, D. G. (1979). J. Dairy Res. 46: 351–355.
Schmidt, D. G., and Payens, T. A. J. (1976). In Surface and Colloid Science, Volume 9 (Matijevic, E., ed.), John Wiley, New York, pp.165–199.
Sreerama, N., and Woody, R. W. (1993). Anal. Biochem. 209: 32–44.
Stapley, B. J., and Creamer, T. P. (1999). Protein Sci. 8: 587–595.
Swaisgood, H. E. (1992). In Advanced Dairy Chemistry: Proteins (Fox, P. F., ed.), Elsevier Science Publishers, London, New York, pp. 63–111.
Syme, C. D., Blanch, E. W., Holt, C., Jakes, R., Goedert, M., Hecht, L., et al. (2002). Eur. J. Biochem. 269: 148–156.
Tai, M., and Kegeles, G. (1984). Biophys. Chem. 20: 81–87.
Takase, K., Niki, R., and Arima, S. (1980). Biochim. Biophys. Acta 622: 1–8.
Thompson, M. P. (1966). J. Dairy Sci. 49: 792–795.
Thurn, A., Burchard, W., and Niki, R. (1987). Polymer Sci. 265: 653–666.
Tiffany, M. L., and Krimm, S. (1968). Biopolymers 6: 1379–1382.
Tompa, P. (2002). Trends Biochem. Sci. 27: 527–533.
Torii, H., and Tasumi, M. (1998). J. Ramam Spectrosc. 29: 81–86.
Toumadje, A., and Johnson, W. C., Jr. (1995). J. Am. Chem. Soc. 117: 7023–7024.
Uversky, V. N. (2002). Eur. J. Biochem. 269: 2–12.
Wahlgren, N. M., Dejmek, P., and Drakenberg, T. (1994). J. Dairy Res. 61: 495–506.
Waugh, D. F., Creamer, L. K., Slattery, C. W., and Dresdner, G. W. (1970). Biochemistry 9: 786–795.
Wilder, C. L., Friedrich, A. D., Potts, R. O., Daumy, G. O., and Francoeur, M. L. (1992). Biochemistry 31: 27–31.
Wyman, J., Jr. (1964). Adv. Protein Chem. 19: 223–286.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Qi, P.X., Wickham, E.D. & Farrell, H.M. Thermal and Alkaline Denaturation of Bovine β-Casein. J Protein Chem 23, 389–402 (2004). https://doi.org/10.1023/B:JOPC.0000039553.66233.3f
Issue Date:
DOI: https://doi.org/10.1023/B:JOPC.0000039553.66233.3f