Abstract
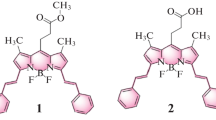
A group of structurally rigid analogues of 2,6-distyrylpyridine was synthesised. Molecular geometry of the synthesised dyes in solutions was studied by 1 H-NMR, electronic absorption and fluorescence spectrometry. The spectral data testify all the compounds exist in E-configuration of their styryl residues. The most planar molecular conformation is typical for the compounds with five-membered side aromatic moieties. In the case of pyridines with six-membered aromatic residues steric hindrance results in turning the above mentioned cyclic groups out of the plane of the central pyridine moiety. The violation of planarity in this case is not significant and saves the possibility of π-electronic conjugation in the molecules. The synthesised compounds are characterized by high fluorescence quantum yields in solutions. The electronic absorption spectra of titled pyridines demonstrate low sensitivity to the nature of the substituents introduced into the side aromatic rings. In contrast to this, the fluorescence bands considerably change their position under the influence of electron donor substituents. The fluorescence spectra display substantial positive solvatofluorochromism only in the cases of the dialkylamino-derivatives, especially on going from aprotic solvents to proton donor ones. Generally, the synthesised structurally rigid distyrylpyridine derivatives have prospects for their application as multi-purposes fluorescent probes.
Similar content being viewed by others
References
B. M. Krasovitskii and B. M. Bolotin (1988). Organic Luminescent Materials, VCH GmbH, Weinheim, Germany.
A. P. De Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher, and T. E. Rice (1997). Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 97, 1515-1566.
B. Valeur and I. Leray (2000). Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev. 205, 3-40.
R. P. Haugland (1998). Handbook of Fluorescent Probes and Research Products. 8th ed., Molecular Probes, Inc.
A. O. Doroshenko, A. V. Grigorovich, E. A. Posokhov, V. G. Pivovarenko, and A. P. Demchenko (1999). Bis-azacrown derivative of di-benzylidene-cyclopentanone as alkali earth ion chelating probe: Spectroscopic properties, proton accepting ability and complex formation with Mg2+ and Ba2+ ions 1. Mol. Eng. 8, 199-215.
A. O. Doroshenko, A. V. Grigorovich, E. A. Posokhov, V. G. Pivovarenko, and A. P. Demchenko (2001). Complex formation between azacrown derivatives of dibenzylidenecyclopentanone and alkali-earth metal ions. Russ. Chem. Bull., Intl. Edn. 50(3), 404-412.
V. G. Pivovarenko, A. V. Klueva, A. O. Doroshenko, and A. P. Demchenko (2000). Bands separation in fluorescence spectra of ketocyanine dyes: Evidence for their complex formation with monohydric alcohols. Chem. Phys. Lett. 325, 389-398.
V. Baliah and R. Jeyaraman (1977). 8-Aryl-3,5-diarylidene-1,2,6,7-tetrahydrodicyclopenta-[b,e]pyridines by the condensation of cyclopentanone with substituted benzaldehydes in the presence of ammonium acetate. Indian J. Chem. B 15, 797-799.
K. Ganapathy and R. Jeyaraman (1979). Mass spectral studies of 8-aryl-3,5-diarylidene-1,2,6,7-tetrahydrodicyclopenta[b,e]pyridines. Indian J. Chem. B 17, 389-390.
R. Jeyaraman and S. Avila (1981). Chemistry of 3-azabicyclo[3.3.1]nonanes. Chem. Rev. 81, 149-174.
D. M. Kneeland, K. Ariga, V. M. Lynch, Ch.-Yu. Huang, and E. V. Anslyn (1993). Bis(alkylguanidinium) receptors for phospohodiesters: Effect of counterions, solvent mixtures, and cavity flexibility on complexation. J. Am. Chem. Soc. 115, 10042-10055.
M. J. S. Dewar, E. G. Zoebich, E. F. Healy, and J. J. P. Stewart (1985). AM1: A new general purpose quantum mechanical molecular model. J. Am. Chem. Soc. 107, 3902-3908.
A. J. Gordon and R. A. Ford (1972). The Chemist's Companion. A Handbook of Practical Data, Techniques and References, Wiley-Interscience, New York.
W. A. Melhuish (1961). Quantum efficiencies of fluorescence of organic substances effect of soluent and concentration of the fluorescent solute. J. Phys. Chem. 65(2), 229-238.
J. N. Demas and G. A. Crosby (1971). Measurement of photoluminescence quantum yields [Review]. J. Phys. Chem. 75, 991-1025.
A. O. Doroshenko, A. V. Kirichenko, V. G. Mitina, and O. A. Ponomaryov (1996). Spectral properties and dynamics of the excited state structural relaxation of the ortho analogues of POPOP-effective abnormally large Stokes shift luminophores. J. Photochem. Photobiol. A: Chem. 94,15-26.
H. Günther (1984). NMR Spectroscopy. An Introduction, Wiley, Chichester, NY.
M. V. Barnabas, A. Liu, A. D. Trifunac, V. V. Krongauz, and C. T. Chang (1992). Solvent effects on the photochemistry of a ketocya-nine dye and its functional analogue, Michler's ketone. J. Phys. Chem. 96, 212-217.
Z. R. Grabowski, K. Rotkiewicz, A. Semiarczuk, D. J. Cowley, and W. Baumann (1979). Twisted intramolecular charge transfer states (TICT). A new class of excited states with a full charge separation. Nouv. J. Chim. 3(7), 443-453.
E. Lippert, W. Rettig, V. Bonacic-Koutecky, F. Heisel, and J. A. Miehe (1987). Photophysics of internal twisting. Adv. Chem. Phys. 68,1-98.
P. Borowicz, J. Herbich, A. Kapturkiewicz, M. Opallo, and J. Nowacki (1999). Radiative and nonradiative electron transfer in donor-acceptor phenoxazine and phenothiazine derivatives. Chem. Phys. 249, 49-62.
V. M. Feygelman, J. K. Walker, A. R. Katrizky, and Z. Deda-Szafran (1989). Studies of sterically hindered oxadiazoles as potential fluo-rescent dopants for polymeric scintillators. Chem. Scr. 29, 241-243.
F. Vollmer, W. Rettig, and E. Birckner (1994). Photochemical mech-anisms produsing large fluorescence stokes shofts. J. Fluoresc. 4(1), 65-69.
A. O. Doroshenko, A.V. Kyrychenko, and J. Waluk (2000). Low temperature spectra of the ortho-POPOP molecule: Additional arguments of its flattening in the excited state J. Fluoresc. 10(1), 41-48.
A. O. Doroshenko, A. V. Kyrychenko, V. N. Baumer, A. A. Verezubova, and L. M. Ptyagina (2000). Molecular structure, fluorescent properties and dynamics of excited stste structural relaxation of the oxadiazolis otrho-analog of POPOP with the additional sterical hindrance. J. Mol. Str. 524, 289-296.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pivovarenko, V.G., Grygorovych, A.V., Valuk, V.F. et al. Structurally Rigid 2,6-distyrylpyridines—A New Class of Fluorescent Dyes. 1. Synthesis, Steric Constitution and Spectral Properties. Journal of Fluorescence 13, 479–487 (2003). https://doi.org/10.1023/B:JOFL.0000008058.34149.df
Issue Date:
DOI: https://doi.org/10.1023/B:JOFL.0000008058.34149.df